BACKGROUND: Participating in early phase clinical trials is encouraged among patients who have exhausted standard treatment options. There is continued focus at the federal level to develop programs addressing racial disparities. Data on disparities in solid tumors such as lung, breast, and colorectal cancers is vast. However, racial and ethnically based outcomes in hematologic malignancies, early phase clinical trial participation and observed clinical outcomes from these trials have received much less attention.
AIM: To describe, evaluate and compare the clinical outcomes and characteristics of a cohort of consecutive Hispanic and Non-Hispanics patients with advanced hematologic malignancies enrolled on early phase clinical trials at the Cancer Therapy and Research Center, (CTRC) from 2000 to 2013.
METHODS: We retrospectively reviewed records of patients with advanced hematological malignancies treated within phase I/II clinical trials at the CTRC and report descriptive statistics used to summarize patients' baseline characteristics on demographics, response, toxicities and survival based on ethnicity. Categorical data were described with contingency tables including counts. The t- and chi square tests were used to compare the differences in survival (PFS and OS) and toxicity between ethnicities. This report constitutes the preliminary findings of the first 106 patients out of a larger cohort of patients currently under study.
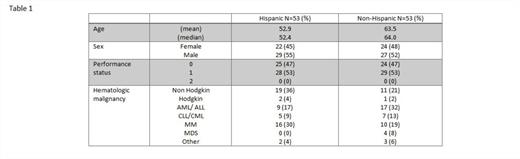
RESULTS: One hundred and six patients were treated. The median number of prior systemic therapies was 3 for both Hispanics (range 0-5) and Non-Hispanics (range 0-12). The median age of Hispanic patients was 52 years and for non-Hispanics was 64 years, with a male/female ratio of 29/22 for Hispanics and of 27/24 for non-Hispanics respectively. (Table1)
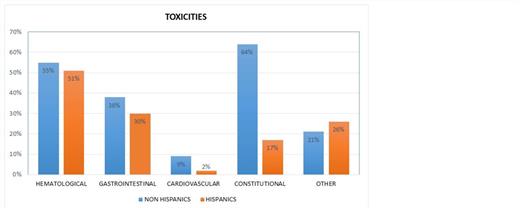
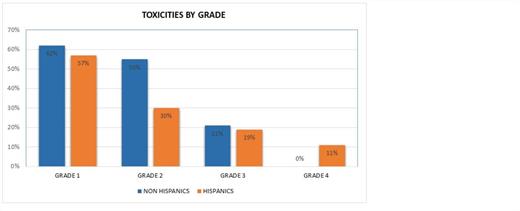
Among 53 Hispanic patients, the mean progression free (PFS) and overall survival (OS) were 3.6 and 5.3 months respectively [95% CI for PFS 2.45-4.6 months and for OS 3.5–7.1 months]. Among 53 Non-Hispanic patients, the mean PFS and OS were 3.9 and 5.2 months respectively [95% CI for PFS 2.4-5.2 months and for OS 2.7–7.7 months]. There were no statistically significant differences in outcome between ethnicities. (p=0.73 for PFS and p=0.97 for OS). The derived clinical benefit rate (CBR=SD+CR+PR) was 26% for Hispanics and 22% for Non-Hispanic patients. Significant difference was observed in the rates of G3/G4 toxicities. (figure1) Sixteen Hispanic patients (30%) vs. 11 non-Hispanic patients (21%) developed grade 3 or 4 toxicity (p=0.02).
CONCLUSION:Our preliminary clinical outcome and safety data suggests that early phase clinical trials may offer similar potential clinical benefit for Hispanic patients with advanced hematological malignancies when compared with those of non-Hispanic origin. It appears that there was a slight increased risk of grade 3 and 4 toxicities when compared with patients of non-Hispanic origin. Further analysis is ongoing to determine prognostic indicators in this population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal