Abstract
Introduction: Double-unit CBT (DCBT) in adult patients (pts) with hematologic malignancies has been associated with high rates of disease-free survival (DFS) but its role in children is controversial.
Methods: We investigated DCBT in children with high-risk acute leukemia following TBI or chemotherapy-based myeloablative cytoreduction. Outcomes of consecutive DCBT pts transplanted 10/2005-2/2013 as their first allograft were evaluated.
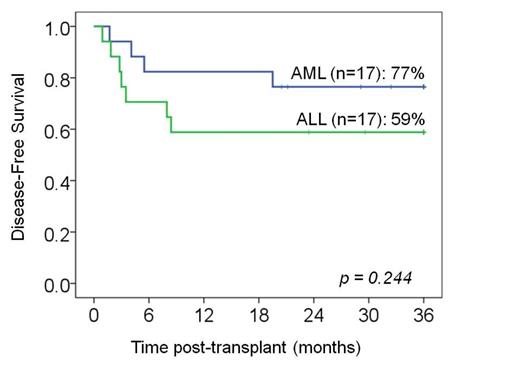
Results: Thirty-five pts [median age 7.5 yrs (range 0.8-18), median weight 28 kg (range 8-75)] were transplanted. Seventeen had acute myelogenous or biphenotypic leukemia (AML): 6 in CR1 (one each with M7, secondary 5q- MDS, FLT-3 ITD mutation, Ph+, Down syndrome with positive MRD, or germline mutation CEBPa), 8 in CR2 (one with MLL positive MRD), 2 in aplasia, and one in CR3. Seventeen pts had ALL: 10 in CR1 [3 Ph+ (one with MRD), 2 T-cell ALL, one MLL, one L3 disease, and 3 multiple inductions], 4 in CR2, and 3 in CR3. One pt had CML (imantinib resistant, accelerated phase with MRD). Thirty-one percent were CMV seropositive and 69% had non-European ancestry. Conditioning was cyclophosphamide/fludarabine/TBI 1375 cGy (N=21, 60%), or in the very young or those with prior radiation a chemotherapy-based regimen was used (N=14, 40%, 10 with clofarabine/thiotepa/melphalan and 4 with busulfan/melphalan/thiotepa). GVHD prophylaxis was with calcineurin-inhibitor/mycophenolate mofetil. Units had a donor-recipient 4-6/6 HLA-A,-B antigen,-DRB1 allele match, a cryopreserved total nucleated cell (TNC) dose >1.5 x 107/kg/unit, and were albumin reconstituted for pts >20 kg or washed for smaller pts. The cumulative incidence of sustained donor neutrophil engraftment was 94% (95%CI:78-98, median 21 days, range 12-33) and hematopoiesis was mediated by a single unit. Day 180 platelet engraftment >50 x 109/l was 82% (95%CI: 64-92). The median platelet recovery in 31 evaluable pts was 51 days (range 39-299). Immune recovery was prompt with a mean absolute CD4+ count of 201 (SD:+/-180) at day +60, and 250 (SD:+/-150) at day +120. The engrafting unit had a median infused TNC dose of 3.9 x 107/kg (range 0.9-12.8) and 10/33 (30%) pts engrafted with a unit that had a pre-cryopreservation TNC <2.5x107/kg. In addition, the majority (17/33, 51%) of pts engrafted with a unit that was <5/8 HLA-allele matched to the recipient (range 2-5/8). The cumulative incidence of day 100 grade II-IV acute GVHD was 46% (95%CI:29-61) and 23% (95%CI:11-38) of pts had grade III-IV acute GVHD. The 3-year incidence of chronic GVHD was 14% (95%CI:5-28). With a median survivor follow-up of 58 months (range 20-105), the 3-year cumulative incidences of transplant-related mortality (TRM) and relapse were 11% (95%CI:4-24) and 20% (95%CI:9-35), respectively. Transplant-related causes of death were 2 graft failures, 1 HHV-6 encephalitis (day +53) and 1 RSV/metapneumovirus pneumonia (day +28). While some pts with GVHD required prolonged immunosuppressive therapy, none died of GVHD. Of the 7 children with relapse, 2 had AML in CR1 (one FLT-3 ITD mutation, one M7 AML), one had primary refractory AML transplanted in aplasia, and 4 had ALL (2 CR1, 1 CR2, 1 CR3). None of the 4 pts transplanted with MRD relapsed. Three-year DFS was 68% (95%CI:50-81). There was no difference based on diagnosis (3-yr DFS 77% in AML and 59% in ALL, p = 0.25, Figure), TBI-based cytoreduction (p = 0.68), or European vs non-European ancestry (p = 0.24). Positive recipient CMV serostatus was associated with lower DFS in univariate analysis (p = 0.005) with 5/11 CMV+ pts relapsing.
Conclusions: Despite high-risk disease and grafts with a very high degree of donor-recipient HLA-allele mismatch, the low TRM and relapse rates after pediatric DCBT are striking. Although many of the younger children could have had “adequate” single unit grafts based on the recently published CIBMTR definition (cryopreserved TNC >3.0 x 107/kg and 6-8/8 allele HLA-match), a significant minority will not. Therefore, despite the lack of benefit of DCBT in the BMT CTN randomized study, DCBT remains an important consideration in children, especially in those of non-European ancestry. Finally, chemotherapy-only-based conditioning is an effective alternative to high-dose radiation, an approach that further extends transplant access to pts unsuitable for TBI.
Boulad:Genzyme Sanofi: Trials partially funded by Genzyme Sanofi Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal