Abstract
Objective: Joint damage due to recurrent joint bleeds remains the most common complication in hemophilia. The combination of cartilage degeneration and synovial inflammation ultimately lead to hemophilic arthropathy. Cartilage destruction is considered to result from both cartilage matrix-degrading proteases as well as cartilage-destructive pro-inflammatory cytokines such as interleukin (IL)-1b. To unravel the role of IL1b in the pathogenesis of blood-induced cartilage damage, we investigated whether direct blocking of IL1b specifically prevents blood-induced cartilage damage in vitro. Moreover, we investigated whether blocking IL1b after the onset of a bleed can still avert cartilage damage.
Methods: Full thickness healthy human articular cartilage explants, obtained post-mortem, were cultured for four days in presence or absence of 50% v/v whole blood. A recombinant human IL1b monoclonal antibody (IL1bmAb) was added at the moment of blood exposure in a concentration of 0, 1, 3, 10, 30, or 100ng/mL (n=8). Furthermore, 100ng/mL IL1bmAb was administered directly or after a delay of several hours up to two days (n=7). Cartilage matrix proteoglycan turnover was determined 12 days later to analyse long-term effects.
To investigate the direct effects of IL1bmAb on cartilage, explants were cultured for four days in the presence of 10ng/mL IL1bmAb in the absence of blood (n=7).
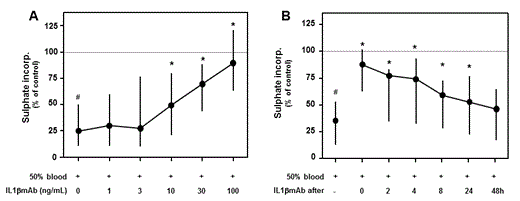
Results: Exposure to blood decreased the proteoglycan synthesis rate and -content, and increased the proteoglycan release (all p<0.05). Adding IL1bmAb resulted in a dose-dependent increase of the proteoglycan synthesis rate leading to normalisation at higher concentrations (see figure A). Proteoglycan release and content also normalised after addition of IL1bmAb. Its protective effect was most pronounced when IL1bmAb was administered within 4 hours after the bleed, but significant recovery was still achieved by administration within 8-24 hours (see figure B for proteoglycan synthesis). In the absence of blood, IL1bmAb did not have direct effects on cartilage proteoglycan turnover (for all three parameters p>0.50).
Conclusions: This study demonstrates that IL1b is a crucial factor in the development of blood-induced cartilage damage in vitro. Blocking this pro-inflammatory cytokine with a monoclonal antibody protects cartilage from the damaging effects of blood exposure in a dose-dependent way. Early administration after blood-exposure is most beneficial. Further research is warranted to investigate the in vivo capacity of IL1bmAb in prevention and its position as a treatment to limit joint damage upon joint bleeding.
Effect of IL1bmAb on cartilage proteoglycan synthesis rate is dose-dependent (A) and time-dependent (B). Median values ± IQR of at least 7 individual cartilage and blood donors are shown. Hash tags indicate a statistically significant difference from control values, whereas asterisks indicate a statistically significant difference from blood-exposed cartilage without IL1bmAb addition (p<0.05).
Effect of IL1bmAb on cartilage proteoglycan synthesis rate is dose-dependent (A) and time-dependent (B). Median values ± IQR of at least 7 individual cartilage and blood donors are shown. Hash tags indicate a statistically significant difference from control values, whereas asterisks indicate a statistically significant difference from blood-exposed cartilage without IL1bmAb addition (p<0.05).
Figure - Effect of IL1bmAbon cartilage proteoglycan synthesis rate is dose-dependent (A) and time-dependent (B). Median values ± IQR of at least 7 individual cartilage and blood donors are shown. Hash tags indicate a statistically significant difference from control values, whereas asterisks indicate a statistically significant difference from blood-exposed cartilage without IL1bmAb addition(p<0.05).
Schutgens:CSL Behring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal