Abstract
Background:
IO was found to be highly active in pts with refractory-relapsed ALL, with an overall response rate of 58% and a median survival of 6.3 months (mos). Identifying factors associated with different outcomes on IO therapy may help select patients for this treatment and advice of prognosis.
Methods:
A total of 89 pts treated with IO on previous studies were analyzed. IO was given at 1.3-1.8 mg/m2 IV x 1 every 3-4 weeks or weekly (0.8 mg/m2 Day 1, 0.5 mg/m2 Days 8 and 15) every 3-4 weeks. Pretreatment factors associated with achieving marrow complete response (CR) and with survival were analyzed using standard statistical methods.
Results:
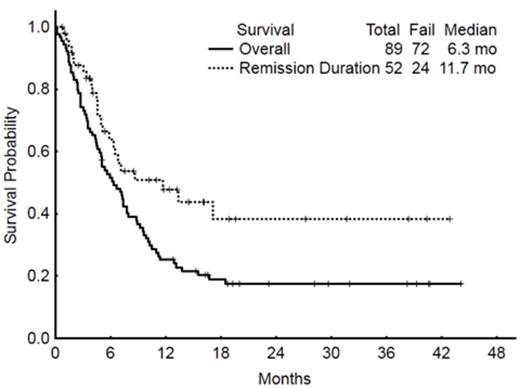
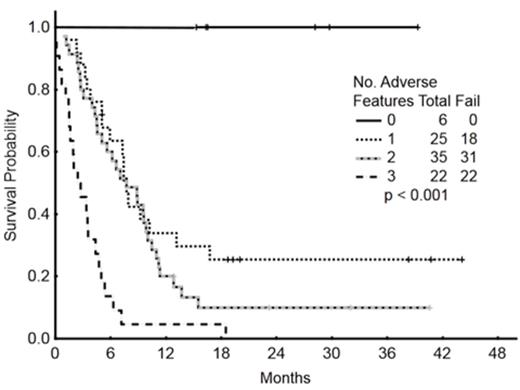
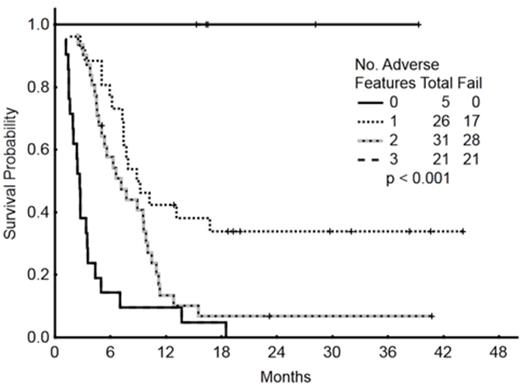
Baseline characteristics of the 89 pts are summarized in Table 1. Overall, 17 pts (19%) achieved CR, 29 (33%) had CRp, and 6 pts (7%) had bone marrow CR with incomplete recovery (Cri). 5 pts (6%) died within 4 weeks of starting therapy. The ORR was 58%. The rate of cytogenetic CR by morphologic responses was as follows: 8 cytogenetic CR/10 morphologic CR (80%); 16 cytogenetic CR/22 morphologic CRp (73%); and 4 cytogenetic CR/4 morphologic CRi (100%). The median survival of patients with at least marrow CR was 9 mos versus 3.4 mos for those without marrow CR (p<0.001). By multivariate analysis, a high peripheral blood absolute blast count (ABC) and low platelet count were independently associated with a lower likelihood of achieving at least marrow CR. With a median follow-up of 23 mos, (5-44), the median overall survival of pts who received IO was 6 mos; the median survival was 5 mos with the single-dose schedule and 9.5 mos with the weekly schedule. The median remission duration was 12 mos (1-year rate, 48%) (Figure 1A). Baseline characteristics independently associated with worse survival included adverse cytogenetics [complex karyotype, translocation (4;11), translocation (9;22), abnormal chromosome 17], disease beyond first salvage, and high peripheral blood ABC. Pts with 0, 1-2 or 3 adverse factors had a median survival of 42+, 7.5, and 2.4 mos, respectively (Figure 1B). To assess the benefit of achieving a marrow CR, we repeated the multivariate survival analysis using a 6-week landmark that excluded 5 pts who died within 6 weeks. The median survival was 9.2 mos and 3.4 mos for patients with and without marrow CR (p<0.001). The multivariate analysis selected the achievement of response as independently associated with survival improvement [HR=0.5 (95% CI=0.28-0.89); p=0.02]. Disease with complex karyotype, translocation (4;11), translocation (9;22), and abnormal chromosome 17 [HR=2.9 (95% CI=1.29-6.62); p=0.009], and disease status beyond first Salvage regimen [HR=1.2 (95% CI=2.16-3.91); p=0.01] were independently associated with significant worse survival. Pts with 0, 1- 2 or 3 adverse factors had a median survival of 42+, 8, and 3 mos, respectively (Figure 1C).
Conclusion:
Our current analyses identified a subset of adult pts with ALL in whom outcome of therapy with IO can be differentially predicted.
Patients' characteristics
| . | . | N (%) . | ||
|---|---|---|---|---|
| Parameter . | . | Single-Dose, n=49 . | Weekly, n=40 . | Overall, n=89 . |
| Age, year | ≤18 | 3 (6) | 3 (8) | 6 (7) |
| ≥60 | 12 (24) | 13 (33) | 25 (28) | |
| ECOG, PS | 0-1 | 44 (90) | 36 (90) | 80 (90) |
| ≥2 | 5 (10) | 4 (10) | 9 (10) | |
| Salvage Status | S1 | 13 (27) | 16 (40) | 29 (33) |
| >S1 | 36 (73) | 24 (60) | 60 (67) | |
| PB ABC, x 109/L | <1.0 | 33 (67) | 25 (63) | 58 (65) |
| ≥1.0 | 16 (33) | 15 (38) | 31 (35) | |
| WBC, x 109/L | <4.0 | 28 (57) | 17 (43) | 45 (51) |
| 4-11 | 11 (22) | 16 (40) | 27 (30) | |
| >11 | 10 (20) | 7 (18) | 17 (19) | |
| BM blasts, % | <20 | 3 (6) | 8 (20) | 11 (12) |
| 20-49 | 10 (20) | 8 (20) | 18 (20) | |
| 50-69 | 8 (16) | 6 (15) | 14 (16) | |
| ≥70 | 28 (57) | 18 (45) | 46 (52) | |
| Platelets, x 109/L | <100 | 43 (88) | 28 (70) | 71 (80) |
| ≥100 | 6 (12) | 12 (30) | 18 (20) | |
| Karyotype | Diploid | 8 (16) | 9 (23) | 17 (19) |
| Ph-positive | 9 (18) | 8 (20) | 17 (19) | |
| Translocation (4;11) | 6 (12) | 3 (8) | 9 (10) | |
| Complex | 13 (27) | 11 (28) | 24 (27) | |
| Abnormal chromosome 17 | 6 (12) | 5 (13) | 11 (12) | |
| Other | 6 (12) | 4 (10) | 10 (11) | |
| Prior ASCT | 7 (14) | 2 (5) | 9 (10) | |
| Prior HCVAD regimen | 35 (71) | 32 (80) | 67 (75) | |
| CD22-positive, % | ≥90 | 28 (57) | 31 (78) | 59 (66) |
| 70-89 | 14 (29) | 7 (18) | 21 (24) | |
| 50-69 | 7 (14) | 2 (5) | 9 (10) | |
| . | . | N (%) . | ||
|---|---|---|---|---|
| Parameter . | . | Single-Dose, n=49 . | Weekly, n=40 . | Overall, n=89 . |
| Age, year | ≤18 | 3 (6) | 3 (8) | 6 (7) |
| ≥60 | 12 (24) | 13 (33) | 25 (28) | |
| ECOG, PS | 0-1 | 44 (90) | 36 (90) | 80 (90) |
| ≥2 | 5 (10) | 4 (10) | 9 (10) | |
| Salvage Status | S1 | 13 (27) | 16 (40) | 29 (33) |
| >S1 | 36 (73) | 24 (60) | 60 (67) | |
| PB ABC, x 109/L | <1.0 | 33 (67) | 25 (63) | 58 (65) |
| ≥1.0 | 16 (33) | 15 (38) | 31 (35) | |
| WBC, x 109/L | <4.0 | 28 (57) | 17 (43) | 45 (51) |
| 4-11 | 11 (22) | 16 (40) | 27 (30) | |
| >11 | 10 (20) | 7 (18) | 17 (19) | |
| BM blasts, % | <20 | 3 (6) | 8 (20) | 11 (12) |
| 20-49 | 10 (20) | 8 (20) | 18 (20) | |
| 50-69 | 8 (16) | 6 (15) | 14 (16) | |
| ≥70 | 28 (57) | 18 (45) | 46 (52) | |
| Platelets, x 109/L | <100 | 43 (88) | 28 (70) | 71 (80) |
| ≥100 | 6 (12) | 12 (30) | 18 (20) | |
| Karyotype | Diploid | 8 (16) | 9 (23) | 17 (19) |
| Ph-positive | 9 (18) | 8 (20) | 17 (19) | |
| Translocation (4;11) | 6 (12) | 3 (8) | 9 (10) | |
| Complex | 13 (27) | 11 (28) | 24 (27) | |
| Abnormal chromosome 17 | 6 (12) | 5 (13) | 11 (12) | |
| Other | 6 (12) | 4 (10) | 10 (11) | |
| Prior ASCT | 7 (14) | 2 (5) | 9 (10) | |
| Prior HCVAD regimen | 35 (71) | 32 (80) | 67 (75) | |
| CD22-positive, % | ≥90 | 28 (57) | 31 (78) | 59 (66) |
| 70-89 | 14 (29) | 7 (18) | 21 (24) | |
| 50-69 | 7 (14) | 2 (5) | 9 (10) | |
Survival by risk score according to a landmark analysis taking into account response as an additional variable
Survival by risk score according to a landmark analysis taking into account response as an additional variable
Kadia:GSK: Research Funding; ARIAD: Honoraria. Hagop:ARIAD: Research Funding; Pfizer: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal