Abstract
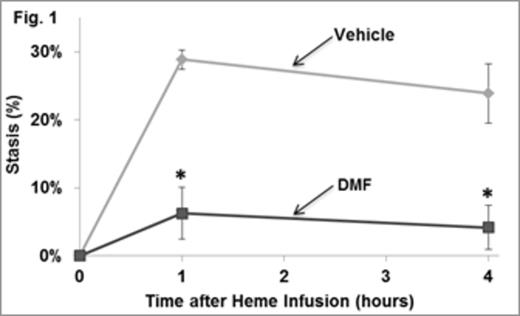
Patients with sickle cell disease have unrelenting hemolysis leading to the release of hemoglobin and heme into the vasculature that promote oxidative stress, inflammation, vaso-occlusive pain crises, ischemia-reperfusion injury and organ damage. Induction of the cytoprotective heme metabolizing enzyme heme oxygenase-1 (HO-1), the iron-binding protein ferritin heavy chain or administration of the HO-1 metabolite CO induces cytoprotective responses that inhibit oxidative stress, inflammation, vaso-occlusion and organ damage in transgenic sickle mice expressing human βS globins. The master regulator of these anti-oxidative and cytoprotective responses is nuclear factor erythroid 2-related factor (Nrf2). Nrf2 activity is controlled, in part, by the cytosolic protein, kelch-like ECH-associated protein 1 (Keap1). Nrf2 is anchored in the cytoplasm through binding with Keap1 which results in its ubiquitination and subsequent proteosomal degradation. Upon exposure to stress stimuli, such as reactive oxygen species and electrophiles, Nrf2 is stabilized and able to translocate to the nucleus where it trans-activates target genes that possess an antioxidant responsive element (ARE) in their promoter regions. Recently the FDA and their European and Canadian counterparts approved dimethyl fumarate (Tecfidera) for the treatment of relapsing multiple sclerosis. Dimethyl fumarate (DMF) and its metabolite monomethyl fumarate alkylate a critical reactive thiol Cys-151 on Keap1 causing release of Nrf2, nuclear localization and activation of cellular anti-oxidant and cytoprotective responses. Based on our previous results showing cytoprotection through HO-1 and it products, we evaluated DMF responses in NY1DD transgenic sickle mice. DMF (15mg/kg) or vehicle (0.08% methyl cellulose) was administered by oral gavage BID X 3 days to NY1DD mice. On the first day of treatment, mice were implanted with dorsal skin-fold chambers. One hour after the last treatment, 20-30 flowing subcutaneous venules were selected and mapped in the dorsal skin-fold chamber window followed by infusion of heme (3.2 µmols/kg) into the tail vein. All of the selected venules were re-examined at 1 and 4 hours post-infusion and the number of static (no flow) venules were counted and expressed as percent stasis. After the 4 hour stasis measurement, blood was collected and organs were removed and flash frozen. In sickle mice treated with vehicle, microvascular stasis was 29% and 24% at 1 and 4 hours, respectively (Fig. 1). In contrast, in sickle mice treated with DMF, stasis was 6% and 4% at 1 and 4 hours (p<0.001). There also was a marked increase in nuclear Nrf2 in liver nuclear extracts of DMF-treated sickle mice on Western blots. Analysis of liver mRNA by qRT-PCR revealed that there was a 2.4 to 4.4-fold enrichment of mRNA coding for putative Nrf2-responsive antioxidant proteins in DMF-treated sickle mice compared to vehicle treated mice (Table 1). This was accompanied by a 3.3-fold increase in HO-1 activity (p<0.05), a distinct increase in cytoplasmic ferritin heavy chain on Western blot and a marked decrease in nuclear NF-κB phospho-p65 activation on Western blot of liver nuclear extracts of DMF treated sickle mice versus vehicle controls. Red blood cells and white blood cells isolated from DMF-treated sickle mice had significantly decreased adhesion to resting and heme- and TNF-activated HUVEC compared to blood cells from mice treated with vehicle. We conclude that DMF may be an effective agent to prevent sickle crises through induction of HO-1, ferritin and other antioxidant proteins, and inhibition of vaso-occlusion. These data combined with prior evidence that monomethyl fumarate can induce hemoglobin F make it an idea candidate for clinical trials in SCD patients.
Enrichment of mRNA for Antioxidant Proteins in DMF-treated Livers
| mRNA . | Fold enrichment . | P value . |
|---|---|---|
| HO-1 | 2.4 | 0.08 |
| Hemopexin | 2.5 | 0.023 |
| Ferritin Heavy Chain | 2.6 | <0.001 |
| Haptoglobin | 2.7 | 0.046 |
| Nrf2 | 2.7 | 0.004 |
| Ferroportin | 3.1 | <0.001 |
| GlutamateCysteine Ligase Catalytic Subunit | 3.3 | <0.001 |
| Glutathione S-Transferase A2 | 3.4 | 0.032 |
| NAD(P)H Dehydrogenase [Quinone] 1 | 3.8 | 0.008 |
| Glutathione S-Transferase Mu 1 | 3.8 | <0.001 |
| Multidrug Resistance-associated Protein 2 | 4.4 | <0.001 |
| mRNA . | Fold enrichment . | P value . |
|---|---|---|
| HO-1 | 2.4 | 0.08 |
| Hemopexin | 2.5 | 0.023 |
| Ferritin Heavy Chain | 2.6 | <0.001 |
| Haptoglobin | 2.7 | 0.046 |
| Nrf2 | 2.7 | 0.004 |
| Ferroportin | 3.1 | <0.001 |
| GlutamateCysteine Ligase Catalytic Subunit | 3.3 | <0.001 |
| Glutathione S-Transferase A2 | 3.4 | 0.032 |
| NAD(P)H Dehydrogenase [Quinone] 1 | 3.8 | 0.008 |
| Glutathione S-Transferase Mu 1 | 3.8 | <0.001 |
| Multidrug Resistance-associated Protein 2 | 4.4 | <0.001 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal