Abstract
Introduction
Despite therapeutic advances in multiple myeloma, disease relapse is common. Combination therapy with dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP) has been utilized as an effective salvage regimen for over a decade, and a recent study reported that DCEP provided an overall response rate of 45.1% when used as salvage therapy in patients who had previously received novel agents (Park S, et al. 2014). Aside from hematologic toxicities, DCEP is generally well-tolerated. In fact, the toxicity profile of DCEP has been compared to high dose cyclophosphamide in the setting of stem cell mobilization and is considered less toxic than the latter. Based upon the synergy noted when proteasome inhibitors are combined with genotoxic therapy, we have combined bortezomib with DCEP in a series of relapsed myeloma patients. Herein we report our experience with the addition of bortezomib to DCEP in relapsed/refractory disease.
Patients and Methods
We performed a retrospective evaluation of patients with relapsed/refractory multiple myeloma treated at Emory University Hospital from October 2011 until March 2014. Patients received dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP) at standard doses in combination with bortezomib at either a dose of 1 mg/m2 or 1.3 mg/m2 administered on Days 1 and 4 of each cycle given every 28 days. Indications for receiving V-DCEP are either cytoreduction prior to SCT (cohort 1) or as salvage therapy (cohort 2).
Results
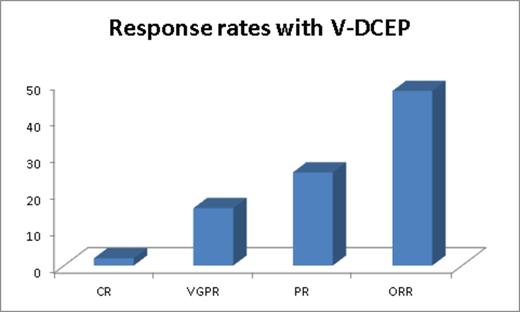
Among the 51 patients (49% male and 51% female) included in analysis, the median age at the time of diagnosis is 58 years (range 30-78) and the time of treatment with V-DCEP is 62 years (range 33-79). Among patients that received V-DCEP as cytoreduction prior to SCT, median prior lines of therapy were 1 (0-8). Among the patients that received V-DCEP as salvage therapy, median number of prior lines of therapy was 3 (1-6). ISS 3 disease was seen in 70% of patients and high risk disease in 72.5% of pts (del 17p: 31%; PCL: 19%; extramedullary disease: 33%; complex CTG: 11%) and t(4;14): 6%). Median time from diagnosis to initiation of V-DCEP therapy among cohort 1 is 18 months (0-86) and among cohort 2 is 31 months (12-105) months. Median serum creatinine before C1D1 is 1.17 (0.61-6) and serum bilirubin is 0.6 (0.1-2.8). 31% of patients needed dose reductions from our standard protocol due to organ dysfunction. 47% of patients received ≥2 cycles. The median time to next cycle is 28 days (20-46) and time to next treatment after V-DCEP is 35 days (25-451) suggesting good hematologic recovery. The overall response rate (≥PR) amongst both cohorts with V-DCEP is 47.8% (40% and 51.6% overall response for cohorts 1 and 2, respectively). Figure 1 illustrates response rates. 10 patients that presented with renal insufficiency had renal response including 2 of the 5 patients on hemodialysis. While the median PFS for cohort 1, as expected has not reached, for cohort 2, it is 8 months (95% CI 5.7-10.3). At a median follow-up of 17 months, from the time of V-DCEP initiation, median OS for cohort 2 is 10 months (95% CI 5-14.9). Median overall survival for this predominantly high risk group of patients from diagnosis in cohort 1 is 78 months (95% CI 47-108) and 49 (95% CI 17-80.7) months in cohort 2, respectively. While only 1 patient with grade 2 peripheral neuropathy (PN) received V-DCEP, change from baseline existing PN was seen in 19% of patients (no grade 3/4 events).
Conclusions
During this era where minimizing alkylator therapy is a consideration, certain indications exist for using V-DCEP such as cytoreduction prior to SCT or as salvage therapy serving as bridge to next line of therapy. Addition of bortezomib to DCEP is deemed safe and is an effective cytoreductive regimen in the treatment of multiple myeloma.
Gleason:Celgene: Consultancy; Novartis: Consultancy. Heffner:Amgen: Honoraria, Research Funding; Biotest: Honoraria, Research Funding; Dana Farber CI: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Idera: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; Onyx: Honoraria, Research Funding; Spectrum: Honoraria, Research Funding; Talon Therapeutics: Honoraria, Research Funding. Lonial:Millennium: The Takeda Oncology Company: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Onyx Pharmaceuticals: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal