Abstract
Introduction: In the randomized placebo controlled phase 3 trial PANORAMA 1 the pan-deacetylase inhibitor (pan-DACi) panobinostat combined with bortezomib and dexamethasone (PAN-BTZ-Dex) led to a statistically significant and clinically relevant increase in progression free survival in patients (pts) with relapsed or relapsed and refractory multiple myeloma (Pbo-BTZ-Dex; 12.0 vs 8.1 months; hazard ratio 0.63, P<0.0001). Gastrointestinal (GI) toxicity, particularly diarrhea, a known class-effect of pan-DACi and BTZ, was the most common non-hematologic adverse event (AE) observed in both treatment arms, occurring at a higher incidence in the PAN-BTZ-Dex arm. Optimal management of GI toxicity, especially diarrhea-related AEs, in pts who receive PAN-BTZ-Dex could help maximize treatment outcomes. Here we present a detailed analysis of pts with diarrhea on the PANORAMA 1 study to inform strategies to reduce the effects of diarrhea and improve efficacy.
Methods: The PANORAMA 1 trial included 12 cycles of treatment with two treatment phases (TP1: eight 3-week cycles; TP2: four 6-week cycles). In TP1, pts were randomized to receive oral PAN (20 mg) or Pbo administered thrice weekly for the first 2 weeks, with intravenous BTZ (1.3 mg/m2) administered on days 1, 4, 8, and 11 and Dex (20 mg) administered orally on the days of and after BTZ. In TP2 (for pts demonstrating clinical benefit), PAN or Pbo were administered similarly but BTZ was administered once-weekly (days 1, 8, 22 and 29) with Dex on the day of and after BTZ. For each cycle, prevalence and incidence for diarrhea AEs were determined; these were characterized by grade, improvement in grade, worsening of grade, and resolution of diarrhea.
Results: In the PANORAMA 1 trial, a total of 260/381 (68.2%) pts treated with PAN-BTZ-Dex and 157/377 (41.6%) pts treated with Pbo-BTZ-Dex reported AEs of diarrhea; most were grade 1 or 2 (PAN-BTZ-Dex: 42.8% vs Pbo-BTZ-Dex: 33.7%). Grade 4 diarrhea was rare (PAN-BTZ-Dex: 1.3%; Pbo-BTZ-Dex: 0.5%). Dose adjustments/interruptions for diarrhea occurred in 26% on the PAN-BTZ-Dex arm and 9% on the Pbo-BTZ-Dex arm. Treatment discontinuation due to diarrhea occurred in 4.5% on the PAN-BTZ-Dex arm and 1.6% on the Pbo-BTZ-Dex arm. Serious AEs of diarrhea occurred in 11.3% on PAN-BTZ-Dex arm and 2.4% on Pbo-BTZ-Dex arm. There was no hemorrhagic diarrhea on the PAN-BTZ-Dex arm and in 1 pt in the Pbo-BTZ-Dex arm.
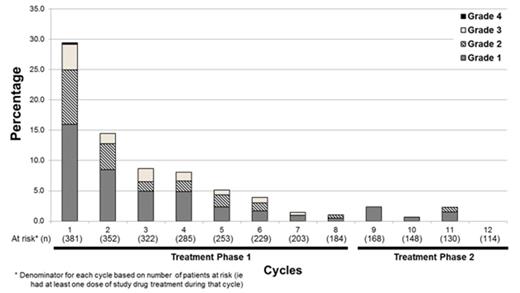
In the majority of pts (56.2%) in the PAN-BTZ-Dex arm, diarrhea occurred for the first time in cycles 1-4 and was primarily grade 1/2; first onset occurred rarely in TP2 (Figure). Cumulative incidence of diarrhea on the PAN-BTZ-Dex arm plateaued by cycle 6 (61.9% by cycle 6; 68.2% overall). The cumulative number of pts with complete resolution of diarrhea (defined as resolved diarrhea that did not recur on treatment) increased over the course of the study. During treatment, 67.7% (258/381) in PAN-BTZ-Dex arm had either no diarrhea or complete resolution of their diarrhea. Analysis of diarrhea management by grade and treatment phase revealed that most pts received medication to manage the diarrhea. Antidiarrheal medications were administered to 70.8% (184/260) pts with diarrhea across all grades; loperamide was used most commonly. However, many pts with grade 1/2 diarrhea had no action taken in TP1: grade 1 - 58% (115/198); grade 2 - 26% (33/127), suggesting suboptimal management of diarrhea.
Conclusions: These data demonstrate that diarrhea in pts who received PAN-BTZ-Dex occurs primarily in the first 1-4 cycles of treatment with cumulative incidence reaching a plateau around cycle 6. Majority of pts with diarrhea had complete resolution during treatment. Although diarrhea was commonly observed in pts who received PAN-BTZ-Dex in the PANORAMA 1 trial, it was manageable and seldom led to treatment discontinuation; however, some pts with grade 1/2 diarrhea did not receive proactive management. Optimal management of diarrhea with prompt intervention including antidiarrheal medication and dose holds/adjustments of BTZ and PAN at the first sign of loose stools may help to maintain pts on therapy and thus improve outcomes with PAN-BTZ-Dex. Future trials should seek to employ these strategies for pts who experience GI toxicity, and should consider subcutaneous administration of BTZ. In addition, studies using combinatorial agents with less overlapping GI toxicity with PAN may further improve safety in this setting.
Richardson:Takeda: Research Funding; Millennium: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Dimopoulos:Celgene: Consultancy, Honoraria. Jedrzejczak:Amgen, Novartis: Consultancy, Research Funding. Guenther:Novartis: Consultancy, Research Funding. Siritanaratkul:Novartis: Research Funding; Roche: Research Funding; Janssen: Research Funding. Schlossman:Millennium: Consultancy. Hou:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees. Moreau:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lonial:Millennium: Consultancy; Celgene: Consultancy; Novartis: Consultancy; BMS: Consultancy; Onyx: Consultancy; Sanofi: Consultancy. Sopala:Novartis Pharma AG: Employment. Panneerselvam:Novartis: Employment, Equity Ownership. Redhu:Novartis: Employment. Corrado:Novartis Pharma AG: Employment. Binlich:Novartis Pharma SAS: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal