Abstract
Background: The association of clinical trial participation with outcomes in cancer patients is controversial, with some studies reporting better outcomes for trial participants while others do not. It is possible that the difference in outcomes is due to differences in baseline characteristics of participants and non-participants. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0201 trial was a large randomized, multi-center study comparing peripheral blood (PB) with bone marrow (BM) for hematopoietic cell transplantation (HCT) from HLA-matched or 1 allele mismatched unrelated donors (URD) in patients with acute leukemia, chronic myeloid leukemia and myelodysplastic syndromes. The trial reported no significant survival differences between PB and BM; there was decreased risk of graft failure but increased risk of chronic graft-versus-host disease (GVHD) with PB grafts (Anasetti et al NEJM 2012).
Methods: We compared characteristics of BMT CTN 0201 study participants (n=494) with HCT recipients who did not participate on the trial (n=1384) but were potentially eligible by virtue of known characteristics. These patients received similar conditioning and GVHD prophylaxis as trial participants at 38 United States trial centers during the study time-period. Centers that had no eligible non-trial participants were excluded. Outcomes between patients on and off trial were compared using Cox proportional hazards regression models. Data were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR) that collects patient data on all trial and non-trial allogeneic HCT recipients through its registry function.
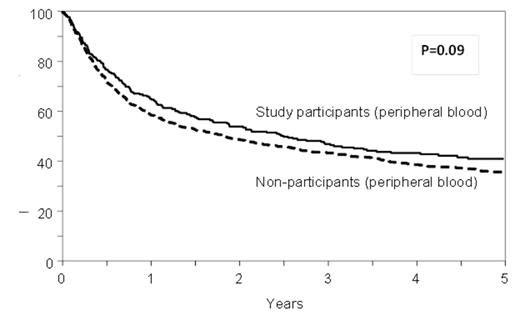
Results: Approximately 29% [494/1878] of apparently eligible patients were randomized and analyzed as part of the trial. There were no significant differences between study participants and non-participants in age or sex distribution, disease types, HLA or sex matching, comorbidities, or interval from diagnosis to HCT. Higher proportions of non-participants were Hispanic, had a lower performance status and lower disease risk, and received myeloablative conditioning and antithymocyte globulin (ATG) or Campath than the study participants. PB was used more commonly as the graft source in the non-participants as compared to the study participants (65% vs. 50%, p<0.001). In multivariate models, overall mortality (HR 1.09, 95% CI 0.95-1.25, p=0.22), transplant-related mortality (HR 1.08, 95% CI 0.89-1.32, p=0.42) and risks of acute grade II-IV (HR 0.96, 95% CI 0.82-1.12, p=0.57) and chronic GVHD (HR 1.06, 95% CI 0.92-1.23, p=0.41) were comparable between study non-participants and participants. Non-participants were at higher risk for relapse than participants (HR 1.24, 95%CI 1.04-1.49, p=0.02) in the multivariate analysis adjusted for disease risk and other clinical variables. Center effect was examined and was not found to be significant (p=0.10). There was no significant interaction between graft source and trial participation (P=0.22) and the risk of mortality was comparable for BM (HR 0.99, 95% CI 0.80-1.22, p=0.90) and PB recipients (HR 1.17, 95% CI 0.97-1.41, p=0.09) whether on trial or off trial. (Figure)
Conclusion: Approximately 29% of patients who appeared eligible and were treated with conditioning and GVHD prophylaxis regimens allowed on the trial were enrolled. Despite some differences in clinical characteristics, most of the outcomes of non-trial participants were comparable to trial participants, suggesting that there is no definitive evidence that the outcomes of patients in a clinical trial are superior to those of the non-participants treated in a similar fashion. The comparable outcomes also indicate that the results of this trial were not seriously affected by selection bias and are therefore representative of the larger population.
Adjusted overall survival in BMT CTN 0201 trial participants and non-participants
Adjusted overall survival in BMT CTN 0201 trial participants and non-participants
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal