Abstract
Background: Essential thrombocythemia (ET) is a Philadelphia chromosome–negative myeloproliferative neoplasm (MPN) characterized by persistent thrombocytosis, excessive proliferation of megakaryocytes in the bone marrow, and normal erythrocyte mass. As with the other Philadelphia chromosome–negative MPNs, myelofibrosis (MF) and polycythemia vera (PV), ET is associated with dysregulated Janus kinase (JAK)-signal transducer and activator of transcription signaling. Ruxolitinib is an oral JAK1/JAK2 inhibitor that has shown clinical benefit in patients with MF and PV.
Objective: To report the long-term efficacy and safety of ruxolitinib in patients with ET from an open-label, phase II study (INCB18424-256; NCT00726232) in patients with ET or PV. Long-term results from the patients with PV have been published (Verstovsek S et al, Cancer. 2014;120:513-520).
Methods: Eligible patients had ET refractory to or intolerant of hydroxyurea as assessed by the investigator, platelet count > 650 × 109/L unless receiving treatment, and absolute neutrophil count ≥ 1.2 × 109/L. Efficacy endpoints included achievement of platelet counts ≤ 400 × 109/L in patients with baseline counts > 400 × 109/L, platelet count ≤ 600 × 109/L in patients with baseline counts > 600 × 109/L, white blood cell (WBC) counts ≤ 10 × 109/L in patients with baseline counts > 10 × 109/L, and a ≥ 50% reduction in palpable splenomegaly in patients with a palpable spleen at baseline. Patients assessed their ET-related symptoms (pruritus, bone pain, night sweats, asthenia, and paresthesia) on a scale from 0 (not present) to 10 (worst imaginable) at each study visit, reporting the worst level of symptoms experienced during the 7 days preceding the study visit. Patients who discontinued were counted as nonresponders for all response analyses.
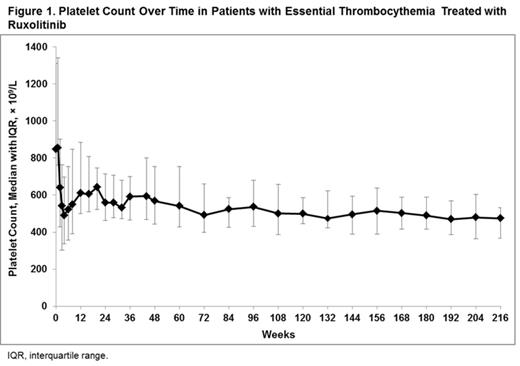
Results: At baseline (N = 39), median age was 51.0 years, median disease duration was 87.6 months, median hematocrit was 40.4%, median platelet count was 849.0 × 109/L, and median WBC was 8.15 × 109/L. At the time of data cutoff, the median exposure to ruxolitinib was 205.6 weeks (approximately 4 years; median total daily dose 30.1 mg) and 61.5% of patients were still receiving treatment. The majority of patients (71.8%) received ruxolitinib for ≥ 96 weeks (24 months). Platelet count decreased rapidly after initiation of therapy and remained relatively stable over time (Figure 1). Of the patients with baseline platelet count > 400 × 109/L (n = 38), 2.6%, 10.5%, and 13.2% of patients had a platelet count ≤ 400 × 109/L at week 12, 48, and 192 (month 3, 12, and 48), respectively. Of the patients with baseline platelet count > 600 × 109/L (n = 35), 48.6%, 40.0%, and 57.1% of patients had a platelet count ≤ 600 × 109/L at week 12, 48, and 192, respectively. Of the patients with baseline WBC count > 10 × 109/L (n = 11), 100%, 72.7%, and 72.7% of patients had a WBC count ≤ 10 × 109/L at week 12, 48, and 192, respectively. Of the 4 patients with palpable spleen at baseline (median 5.0 cm), all patients achieved ≥ 50% reduction and 3 patients achieved absence of palpable splenomegaly by the week 36 visit. Rapid reductions in pruritus, bone pain, night sweats, asthenia, and paresthesia symptom scores were reported by patients during the study, which were largely sustained through week 192 (Figure 2). New or worsening grade 3 or 4 leukopenia, neutropenia, and lymphopenia occurred in 3 patients each (7.7%), and new or worsening grade 3 anemia occurred in 1 patient (2.6%). The most common nonhematologic adverse events of any grade regardless of causality were increased weight (35.9%), diarrhea (28.2%), cough (25.6%), and headache (25.6%). Most adverse events were grade 1 or 2. Infections of any grade were reported in 26 patients (66.7%); of these, grade ≥ 3 infections were reported in 2 patients (5.1%; n = 1, bronchitis and n = 1, pneumonia). No patients experienced transformation to post-ET MF or acute myeloid leukemia.
Conclusions: Ruxolitinib treatment was generally well tolerated in this population of patients with ET who were refractory or intolerant to hydroxyurea and resulted in improvements in platelet count, WBC count, splenomegaly, and disease-related symptoms.
Verstovsek:Incyte: Research Funding. Off Label Use: Ruxolitinib is approved for the treatment of patients with intermediate or high risk myelofibrosis in the United States. Ruxolitinib is not approved for the treatment of essential thrombocythemia. . Rambaldi:Amgen: Consultancy. Zhang:Incyte Corporation: Employment. Vannucchi:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal