Abstract
Background. Polycythemia vera (PV) and essential thrombocythemia (ET) are chronic myeloproliferative neoplasms that can progress to secondary myelofibrosis (MF), named post-PV (PPV) MF and post-ET (PET) MF. While outcome of primary myelofibrosis is defined on the basis of the IPSS models, information on outcome of secondary myelofibrosis (SMF) is scant. The objective of this study is to develop a prognostic model to predict survival of SMF.
Patients and Methods.A total of 718 patients from 16 international Centers were included in this study. Diagnosis of PPV-MF and PET-MF was according to the IWGMRT criteria. Since survival varied among centers, statistical analyses took into account this cluster effect. Survival was calculated utilizing Kaplan-Meier estimates for univariate categorical risk factors and Cox proportional hazard regression, when performing multivariate analysis. The log-rank test was applied to compare Kaplan-Meier curves. Survival was censored at date of stem cell transplant, splenectomy or trial enrollment.
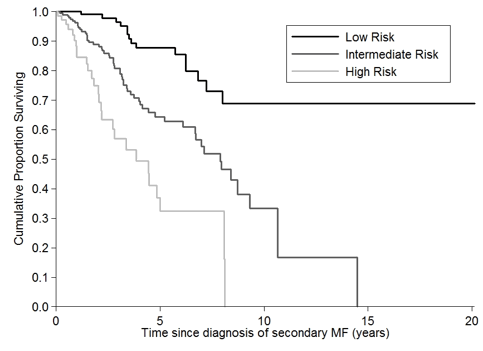
Results. Among 718 SMF, 364 (51%) were PET-MF and 354 (49%) PPV-MF. The median follow-up was 2.4 years (range, 0.6-20.1). Median age was 64 years (range, 25-96), 370 were males (52%). Median time from PV/ET diagnosis to SMF diagnosis was 10.6 years (range, 2.5-41). Median values of hemoglobin, white blood cell count and platelet count were 11.2 g/dL (range, 5-17), 10.2 x10(9)/L (range, 1.1-98.4) and 343 x10(9)/L (range, 16-1908), respectively. Splenomegaly was present in 606 patients (87%) and constitutional symptoms in 329 (47%). Cytogenetic profile (N= 338) was normal in 222 (66%), unfavorable in 40 (12%), abnormal but favorable in 76 (22%), and the JAK2 (V617F) mutation (N=679) in 73%. Anemia, leukocytosis, thrombocytopenia, advanced age, circulating blasts, JAK2(V617F), constitutional symptoms, time to SMF, history of thrombosis negatively affected survival in univariate analysis (maximum P values, 0.043), while type of diagnosis (PPV, PET), splenomegaly, spleen size, cytogenetics, and cardiovascular risk did not. Since survival did not differ between PPV and PET, multivariable analysis was carried out on a subset of 493 SMF patients randomly selected and stratified by diagnosis from the original cohort of 718 patients to generate the MYSEC-PM. The remaining 225 were used as validation cohort. By multivariable analysis, age over 65 years (Hazard Ratio –HR- 2.6, 95%CI: 1.5-4.4; p<0.0001), time from PV/ET to SMF exceeding 15 years (HR 1.7, 95%CI: 1.3-2.2, p<0.0001), history of thrombosis (HR 2.1, 95%CI: 1.2-3.6, p=0.01), presence of constitutional symptoms (HR 1.8, 95%CI: 1.3-2.6; p=0.001), hemoglobin level less than 10 g/dl (HR 1.5, 95%CI: 1.1-2.0, p=0.004) and circulating blast cells equal to or higher than 1% (HR 2.1, 95% CI: 1.5-2.8, p< 0.0001) retained their significant impact of survival. To develop the prognostic model, we assigned each factor an integer weight closest to the corresponding HR in 421 patients with all available data: age >65 years (3 points), time to SMF >15 years (2 points), history of thrombosis (2 points), constitutional symptoms (2 points), hemoglobin <10 g/dL (1 point) and circulating blast >= 1% (2 points). To facilitate the use in clinical practice, the MYSEC-PM was recoded into three broader categories: low-risk (score, 0 to 2), intermediate-risk (score, 3 to 6), high-risk (score >6). Survival was significantly different among the risk groups (Figure 1, p< 0.0001): median survival and its 95% CI was not reached in low-risk, 7.9 years (95% CI: 6.7-9.3) in intermediate and 3.8 (95% CI: 2.2-5) in high-risk. The predictive value of MYSEC-PM was confirmed in the validation cohort (N=190) with the maximum p value in pairwise comparisons of 0.01. In the validation cohort, by Akaike information criterion, MYSEC-PM model better predicted survival than the IPSS/DIPSS models (N=190, AIC, 330.5 vs. 336.5 vs. 334.3, respectively) and also compared to the DIPSS-plus (N=91, 103.8 vs. 106.5, respectively): lower score implies better prediction.
Conclusions. The MYSEC prognostic model, based on age >65 years, time to SMF >15 years, previous thrombosis, constitutional symptoms, hemoglobin <10 g/dL and circulating blast equal or >1%, clearly distinguishes outcome of patients with SMF and outperforms PMF risk models among those patients. This model can be considered for tailoring treatment according to disease risk in SMF.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal