Abstract
Background. Progression to myelofibrosis (MF) represents a natural evolution of polycytemia vera (PV) and essential thrombocytemia (ET), named post-PV (PPV) MF and post-ET (PET) MF, respectively. Information on secondary MF (SMF) is scant. The objective of this study is to define the clinical phenotype and outcome of SMF on a large number of patients and to investigate differences between PPV-MF and PET-MF.
Patients and Methods. A total of 718 patients with SMF from 16 Centers have been collected. Diagnosis of PPV-MF and PET-MF was made according to the IWGMRT criteria. Differences in the distribution of variables between categories were properly analyzed by Mann-Whitney test or Fisher exact test. The cumulative incidence of thrombosis and of leukemia was estimated with a competing risk approach according to the Kalbfleisch-Prentice method. The study was approved by the Institutional Review Boards of each participating center and conducted in accordance with the principles of the Declaration of Helsinki.
Results. Among 718 SMF, 354 (49%) were PPV-MF and 364 (51%) were PET-MF. Median follow-up from SMF diagnosis was 2.5 (range, 0.6-14.7) years in PET-MF and 2.3 (range, 0.6-20.1) in PPV-MF. Demographics are reported in Table 1. Median time from PV/ET diagnosis to SMF was 10.5 years (range, 0.7-40) in PET-MF and 11.1 years (range, 0.7-41) in PPV-MF. As illustrated in Table 1, patients with PPV-MF have significantly higher leukocyte and hemoglobin, while those with post-ET MF have higher platelet counts. Enlargement of spleen and liver was significantly more frequent in PPV-MF than in post-ET MF as well as the presence of constitutional symptoms. Concerning cytogenetics, we found a higher rate of abnormal karyotype in PPV-MF, but no differences in the rate of unfavorable versus favorable, according to the DIPSS-plus definition (Gangat, JCO 2011). As expected, there was an imbalance in JAK2(V617F) distribution (96.7% in PPV-MF and 47% in PET-MF, p< 0.001).
The 3- and 5-year cumulative incidence of thrombosis, estimated with death as a competing risk,
was 7.5 (95% CI: 4.6-11.3) x100 p/y and 10.4 (95% CI: 6.6-15.1) x100 p/y in PET-MF and 9.7 (95% CI: 6.3-14.1) x100 p/y and 13.1 (95% CI: 8.8-18.2) x100 p/y in PPV-MF without statistically significant difference. The 5- and 10-year cumulative incidence of leukemia, estimated with death as a competing risk was 13.1 (95% CI: 8.8-18.4) x100 p/y and 18.7 (95% CI: 11.8-26.8) x100 p/y in PET-MF and 6.6 (95% CI: 3.9-10.3) x100 p/y and 11.3 (95% CI: 6.2-18) x100 p/y in PPV-MF, resulting in an excess of event in PET-MF (Pepe and Mori test, p=0.001).
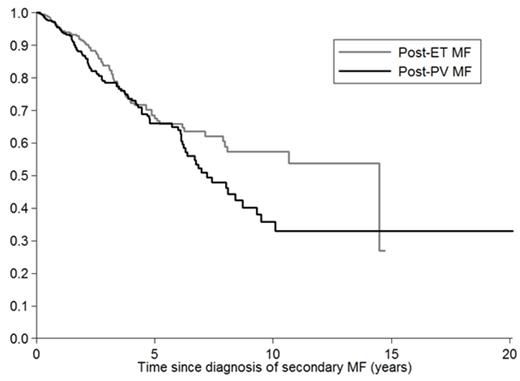
Median survival was 14.5 years (95% CI: 7.9-NR) in PET-MF and 7.2 (95%CI: 6.2-9.3) in PPV-MF, without a statistically significant difference (Figure 1). Survival of patients undergoing treatment was censored at date of hematopoietic stem cell transplant, splenectomy or experimental drug enrollment.
Conclusions. This study on 718 patients with SMF showed that PET-MF and PPV-MF have distinctive clinical phenotypes: more myeloproliferative (leukocytosis, higher hemoglobin levels, splenomegaly, hepatomegaly and symptomatology) in PPV-MF. Leukemia occurred more frequently in PET-MF, while thrombosis incidence was similar. Finally, no differences in survival between PPV-MF and PET-MF have been found.
Demographics of 718 patients with secondary myelofibrosis.
| Variables . | PET-MF (n=364) . | PPV-MF (n=354) . | P value . |
|---|---|---|---|
| Age in years; median (range) | 64 (25-93) | 65 (34-96) | 0.08 |
| Males; n (%) | 175 (48) | 195 (55) | 0.06 |
| Hemoglobin, g/dL; median (range) | 10.7 (5-17.4) | 11.9 (6.8-17.7) | <0.00001 |
| Leukocytes, x109/L; median (range) | 7.95 (1.1-97.3) | 13 (1.7-98.4) | <0.00001 |
| Platelets, x109/L; median (range) | 387 (25-1908) | 293.5 (16-1689) | <0.00001 |
| Circulating blasts; median (range) | 0 (0-19) | 0 (0-13) | 0.8 |
| LDH^ upper the normal range, U/L; n (%) | 228 (82) | 195 (76) | 0.05 |
| Constitutional symptoms; n (%) | 147 (41) | 182 (53) | 0.002 |
| Spleen, cm bcm#; median (range) | 4 (0-27) | 8 (0-34) | <0.00001 |
| Splenomegaly; n (%) | 282 (80) | 324 (94) | <0.0001 |
| Hepatomegaly; n (%) | 89 (29) | 125 (40) | 0.002 |
| Unfavourable cytogenetic/normal karyotype; n (%) n evaluable=320 | 19 (12)/145 (88) | 21 (13)/135 (86) | 0.36 |
| Normal karyotype/abnormal karyotype; n (%) n evaluable=338 | 124 (72)/47 (27) | 98 (59)/69 (41) | 0.005 |
| Time to myelofibrosis in years; median (range) | 10.5 (0.7-40) | 11.1 (0.7-41) | 0.17 |
| Variables . | PET-MF (n=364) . | PPV-MF (n=354) . | P value . |
|---|---|---|---|
| Age in years; median (range) | 64 (25-93) | 65 (34-96) | 0.08 |
| Males; n (%) | 175 (48) | 195 (55) | 0.06 |
| Hemoglobin, g/dL; median (range) | 10.7 (5-17.4) | 11.9 (6.8-17.7) | <0.00001 |
| Leukocytes, x109/L; median (range) | 7.95 (1.1-97.3) | 13 (1.7-98.4) | <0.00001 |
| Platelets, x109/L; median (range) | 387 (25-1908) | 293.5 (16-1689) | <0.00001 |
| Circulating blasts; median (range) | 0 (0-19) | 0 (0-13) | 0.8 |
| LDH^ upper the normal range, U/L; n (%) | 228 (82) | 195 (76) | 0.05 |
| Constitutional symptoms; n (%) | 147 (41) | 182 (53) | 0.002 |
| Spleen, cm bcm#; median (range) | 4 (0-27) | 8 (0-34) | <0.00001 |
| Splenomegaly; n (%) | 282 (80) | 324 (94) | <0.0001 |
| Hepatomegaly; n (%) | 89 (29) | 125 (40) | 0.002 |
| Unfavourable cytogenetic/normal karyotype; n (%) n evaluable=320 | 19 (12)/145 (88) | 21 (13)/135 (86) | 0.36 |
| Normal karyotype/abnormal karyotype; n (%) n evaluable=338 | 124 (72)/47 (27) | 98 (59)/69 (41) | 0.005 |
| Time to myelofibrosis in years; median (range) | 10.5 (0.7-40) | 11.1 (0.7-41) | 0.17 |
^Lactic dehydrogenase
#Below costal margin
Survival of patients with PPV-MF and PET-MF.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal