Abstract
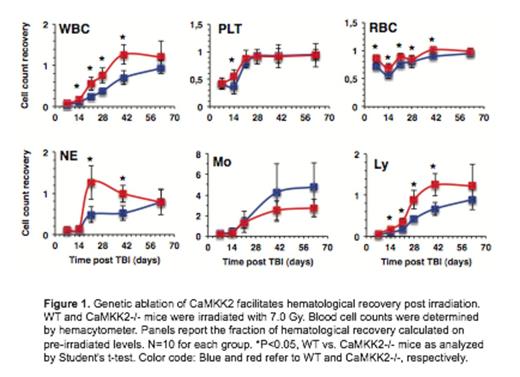
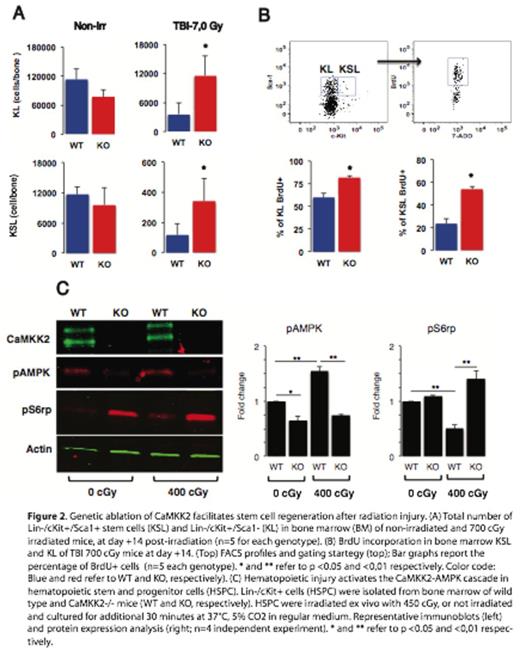
Extracellular free calcium (Ca2+) is the most abundant signaling molecule in the hematopoietic stem cell (HSC) niche, and intracellular calcium is an important second messenger controlling critical functions in HSCs. Accordingly, Ca2+ is a master regulator of HSC biology, and nodes of the Ca2+ signaling network are emerging as critical components of the molecular machinery governing HSC regeneration and quiescence. Ca2+ controls many cell functions by forming a complex with Calmodulin (CaM), which binds and activates a family of Calcium/Calmodulin dependent kinase (CaMK) that includes CaMKI, CaMKII, CaMKIV, CaMKK1 and CaMKK2. We have previously investigated the function of CaMKK2 in blood cells and established this protein as a critical component of the calcium signaling pathway that regulates granulopoiesis and macrophage activation. Here, we investigated the function of CaMKK2 in the regenerative response of HSCs. To determine the expression of CaMKK2 under homeostatic conditions, we examined transgenic reporter mice and identified robust reporter activity in primitive long-term hematopoietic stem cells, but reduced signal in the majority of lineage positive blood and bone marrow cells. To establish whether CaMKK2 has a functional role in controlling hematopoiesis and HSC self-renewal, we examined hematopoietic regeneration in mice deficient for CaMKK2. We demonstrate that Camkk2 -/- mice have accelerated bone marrow and peripheral blood regeneration following hematopoietic injury. Camkk2 -/- HSCs showed increased proliferative capacity in vivo and mobilized into the peripheral blood to establish extra-medullary hematopoiesis during regeneration. To identify the proximal target of CaMKK2, we assessed the phosphorylation status of CaMKI, CaMKIV and adenosine monophosphate activated protein kinase (AMPK), which are known targets of CaMKK2. Our results indicate Camkk2 -/- stem and progenitor cells (HSPC) fail to accumulate phospho-AMPK in response to ionizing radiation. Since AMPK is a negative regulator of the mammalian target of rapamycin (mTOR) pathway, we also analyzed the levels of phospho-ribosomal 6 protein (rpS6), which is a distal target of the mTOR cascade that positively regulates cell size and proliferation. We found that irradiated Camkk2 -/- HSPCs accumulate higher levels of phospho-rpS6 compared to wild type cells. This finding implicates CaMKK2 as a critical component of a negative regulatory circuit that links calcium signaling to AMPK activation and cell proliferation in HSPC. Cumulatively, our data demonstrate that CaMKK2 regulates HSC function and suggests CaMKK2 inhibition is a novel approach to stem cell expansion for clinical therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal