Abstract
Background: Initiation of prophylactic factor VIII (FVIII) therapy at a young age is recommended for the prevention of bleeds and associated arthropathy in severe hemophilia A. Prophylaxis requires frequent infusions of FVIII; children in particular require more frequent infusions due to a shorter FVIII circulating half-life compared with adults. Long-acting FVIII products may allow for prophylaxis with less frequent infusions. Recombinant FVIII Fc fusion protein (rFVIIIFc) had a prolonged half-life, and yielded low annualized bleeding rates (ABR) with once- to twice-weekly prophylaxis in a phase 3 study of adults and adolescents.
Aims: The Kids A-LONG study evaluated the safety, efficacy, and pharmacokinetics (PK) of rFVIIIFc in previously treated children with severe hemophilia A.
Methods: Kids A-LONG was a global, multi-center, open-label, phase 3 study that enrolled males <12 years old with severe hemophilia A (<1 IU/dL endogenous FVIII activity), ≥50 prior exposure days (ED) to FVIII, and no history of FVIII inhibitors. All subjects were to receive twice-weekly prophylactic infusions of rFVIIIFc (25 IU/kg day 1, 50 IU/kg day 4), with adjustments to dosing frequency (up to once every 2 days) and dose (to ≤80 IU/kg) as needed. A subset of subjects (<6 yrs, n = 25; 6 to <12 yrs, n = 35) underwent sequential PK evaluations with their prior FVIII therapy (50 IU/kg) followed by rFVIIIFc (50 IU/kg). The primary endpoint was development of inhibitors. Key efficacy endpoints were ABR and number of infusions required to control a bleed.
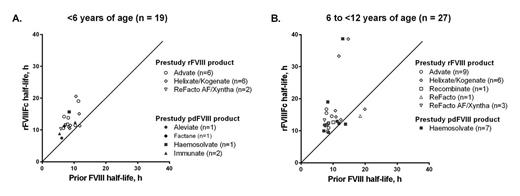
Results: Of the 71 enrolled subjects (<6 yrs, n = 36; 6 to <12 yrs, n = 35), 89% were on a FVIII prophylaxis regimen pre-study; 74.6% of these subjects required ≥3 infusions/week. Advate® was the most commonly reported pre-study product. The median time on study was 26 weeks; 94.4% of subjects completed the study and 61 subjects had ≥50 EDs to rFVIIIFc. No subject developed inhibitors to rFVIIIFc. The pattern of adverse events reported with rFVIIIFc treatment was typical of the population studied. No serious adverse events were assessed by investigators to be related to rFVIIIFc treatment. Two non-serious events (rash and myalgia [muscle pain], reported in 1 subject each) were considered related to rFVIIIFc treatment by investigators. The arithmetic mean (95% Cl) terminal half-life in subjects <6 yrs and 6 to <12 yrs of age was 12.67 hrs (11.23, 14.11) and 14.88 hrs (11.98, 17.77), respectively, based on the one-stage clotting (aPTT) assay. Intra-subject half-life ratios (rFVIIIFc:pre-study FVIII) ranged from 0.79 to 2.98; a plot of ratios by age cohort and product is shown in Fig. 1.
The median on-study dosing interval was 3.5 days, with 89% of subjects receiving rFVIIIFc twice-weekly at study end. Forty-six of 62 subjects (74%) on a prior prophylactic regimen reduced their dosing frequency with rFVIIIFc. Pre-study, 30 subjects were on Advate prophylaxis (twice-weekly, n = 6; ≥3 times/week, n = 24). At study end, 21/24 subjects (88%) previously on ≥3 infusions/week were on a regimen of twice-weekly rFVIIIFc. The median average weekly prophylactic dose on study was 88.1 IU/kg. Table 1 presents the median weekly factor consumption by age cohort among all subjects on pre-study prophylaxis and among subjects on pre-study Advate.
| . | <6 years . | 6 to <12 years . | ||
|---|---|---|---|---|
| Median average weekly consumption (IU/kg/week) | All subjects (n=32) | Subjects on pre-study Advate (n=16) | All subjects (n=30) | Subjects on pre-study Advate (n=14) |
| Prestudy | 98 | 100 | 103 | 106 |
| On-study (rFVIIIFc) | 91 | 97 | 88 | 90 |
| . | <6 years . | 6 to <12 years . | ||
|---|---|---|---|---|
| Median average weekly consumption (IU/kg/week) | All subjects (n=32) | Subjects on pre-study Advate (n=16) | All subjects (n=30) | Subjects on pre-study Advate (n=14) |
| Prestudy | 98 | 100 | 103 | 106 |
| On-study (rFVIIIFc) | 91 | 97 | 88 | 90 |
Median (IQR) on-study ABR was 1.96 (0.00, 3.96) overall and 0.00 (0.00, 0.00) for spontaneous bleeds. In the <6 yrs cohort, median pre-study ABR was 1.50, compared with 0.00 on study; in the 6 to <12 yrs cohort, median pre-study ABR was 2.50, compared with 2.01 on study. 81.4% of all bleeding episodes were controlled with 1 infusion; 93.0% with 1-2 infusions (median average dose per infusion = 49.7 IU/kg).
Summary/Conclusion: In this first phase 3 study of a long-acting FVIII in previously treated pediatric subjects, rFVIIIFc prophylaxis was well-tolerated and no subjects developed inhibitors. In the majority of subjects rFVIIIFc half-life was prolonged relative to FVIII, consistent with the ~1.5-fold increase observed in adults and adolescents. With rFVIIIFc, low bleeding rates were maintained with a reduced infusion frequency compared with prior FVIII therapy, and similar overall factor consumption.
Young:Baxter: Consultancy; Biogen Idec: Consultancy, Honoraria; Kedrion: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy, Honoraria. Mahlangu:Amgen: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biogen Idec: Research Funding, Speakers Bureau; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Biotest: Speakers Bureau. Kulkarni:Baxter: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees; Biogen Idec: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Nolan:Biogen Idec: Research Funding; Bayer: Research Funding. Liesner:Baxter: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Pasi:Bayer: Membership on an entity's Board of Directors or advisory committees; BPL: Membership on an entity's Board of Directors or advisory committees; Biogen Idec: Educational Support and Travel Grants, Educational Support and Travel Grants Other, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Educational Support and Travel Grants, Educational Support and Travel Grants Other; OctaPharma: Educational Support and Travel Grants Other, Membership on an entity's Board of Directors or advisory committees; Pfizer: Educational Support and Travel Grants Other, Membership on an entity's Board of Directors or advisory committees. Barnes:Baxter: Membership on an entity's Board of Directors or advisory committees; Bayer: Educational support and travel grants, Educational support and travel grants Other, Membership on an entity's Board of Directors or advisory committees; Biogen Idec: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Educational support and travel grants, Educational support and travel grants Other, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Educational support and travel grants Other, Honoraria, Speakers Bureau. Neelakantan:Biogen Idec: Employment, Equity Ownership. Gambino:Biogen Idec: Employment, Equity Ownership. Cristiano:Biogen Idec: Employment, Equity Ownership. Barnowski:Biogen Idec: Employment, Equity Ownership. Pierce:Biogen Idec: Employment, Equity Ownership. Allen:Biogen Idec: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal