Abstract
Background: The prothrombotic potential of coagulation factor XI (FXI) is demonstrated by the hypercoagulable state associated with increased plasma levels of FXI and by the risk of thromboembolic events of FXI concentrates. Although inhibition of activated FXI (FXIa) by antithrombotic drugs is emerging as a new approach in anticoagulation, the knowledge about physiological inhibitory mechanisms, which are involved in the control of the activity of FXIa, is limited.
Methods: To evaluate the impact of the SERPINs antithrombin (AT) and C1-inhibitor (C1-INH) on the catalytic life of FXIa, we analyzed inactivation patterns of exogenously added FXIa in normal human plasma, in plasma deficient for the FXIa inhibitors C1-INH and AT, and in a purified system containing different levels of these inhibitors. FXIa was added to the respective sample matrix with a final concentration of 10 ng/mL and endogenous FXIa inactivation stopped by addition of benzamidine. Subsequently, the residual amount of FXIa was quantified by an enzyme capture assay using a monoclonal antibody to capture FXIa. To further evaluate the clinical impact of FXIa inhibitor levels on thrombotic risk, plasma levels of AT, C1-INH, and of the FXIa inhibitors α1-antitrypsin and α2-antiplasmin were measured in a cohort of 98 thrombophilic patients and compared with matched healthy controls.
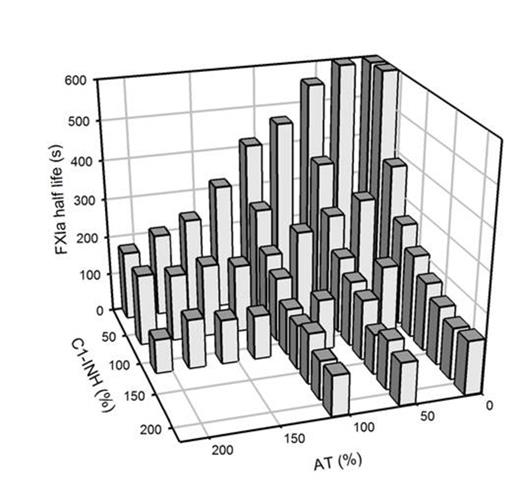
Results: The FXIa inhibition assay demonstrated coefficients of intra- and inter-assay variation of 13.2% and 15.2%, respectively. The catalytic half-life of FXIa in normal human plasma was 133.8 ± 18.8 s (mean ± SD). FXIa half-life times prolonged to 251.6 ± 29.4 s in plasma with decreased activity levels of C1-INH of 25% and to 175.7 ± 3.7 s in AT deficient plasma. After addition of plasma purified C1-INH or AT, FXIa half-life times shortened in a concentration-dependent manner. The dependence of the FXIa inactivation rate on levels of C1-INH and AT was further demonstrated in a purified system as shown in Fig. 1. The observation that AT and C1-INH additively control the catalytic life of FXIa prompted us to measure FXIa inhibitor levels in thrombophilic patients, as a combination of critically low inhibitor levels might constitute a thrombotic risk factor. However, plasma levels of more than one inhibitor below the normal range were not observed in the thrombophilic patients of our study population. Recently, the prothrombotic potential of FXIa was further supported by the detection of residual amounts of FXIa in therapeutic immunoglobulin preparations (IVIG) associated with thromboembolic events. In our system even the presence of high concentrations of IVIG did not affect FXIa inactivation kinetics, making it most unlikely that the prothrombotic potential of IVIG results from impaired FXIa clearance.
Conclusion: The approach utilized in this study allows sensitive and reproducible monitoring of the catalytic life of FXIa in biological sample matrices. The data obtained demonstrate the significant and synergistic contribution of AT and C1-INH to FXIa inactivation in plasma. Critically decreased FXIa inhibitor levels are not a frequent finding in thrombophilic patients.
C1-INH- and AT-dependence of FXIa inactivation rates. Inhibitor levels equivalent to physiological levels in human plasma are referred to as 100%.
C1-INH- and AT-dependence of FXIa inactivation rates. Inhibitor levels equivalent to physiological levels in human plasma are referred to as 100%.
Rühl:Bayer, CSL-Behring: Honoraria, Research Funding. Oldenburg:Baxter, Bayer, Biogen Idec, Biotest, CSL-Behring, Grifols, Novo Nordisk, Octapharma, Swedish Orphan Biovitrum and Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal