Abstract
Background: Eltrombopag (EPAG), an oral thrombopoietin receptor agonist, is approved for treating thrombocytopenia in adults with chronic immune thrombocytopenia (ITP) with insufficient response to prior therapy. Pooled data from 2 similarly designed, randomized, double-blind, placebo (PBO)-controlled studies investigating safety and efficacy of EPAG in pediatric ITP are presented here.
Methods: Subjects aged 1 to <18 years with a confirmed diagnosis of persistent or chronic ITP and a platelet count <30 Gi/L at day 1 were randomized 2:1 to EPAG or PBO and stratified by age: 12–17 years (Cohort 1), 6–11 years (Cohort 2), and 1–5 years (Cohort 3). Subjects could continue baseline ITP medications. After the PBO-controlled randomized phase, subjects were permitted to complete 17 or 24 weeks of treatment with open-label (OL) EPAG. Dose was adjusted based on platelet counts to a maximum of 75 mg daily.
Results: A total of 174 subjects were enrolled in both studies; 171 received ≥1 dose of EPAG. 159 subjects were randomized (intent-to-treat population), and 157 received ≥1 dose of randomized study treatment (safety population). In the randomized period, 3 EPAG and 1 PBO subject discontinued study treatment, of which 2 EPAG and 1 PBO discontinued due to adverse events (AEs). In the OL-EPAG period, an additional 14 EPAG subjects discontinued study treatment, 6 due to AEs. Males comprised 47% of the EPAG and PBO groups and 20% and 24% were East Asians, respectively. Most subjects (93%) were diagnosed with ITP for ≥12 months, and 13% were receiving ITP medications at baseline. The majority of subjects (81%) received ≥2 prior ITP therapies. Most subjects (59%) had a baseline platelet count <15 Gi/L. All 9 (6%) splenectomized subjects were randomized to the EPAG group.
Randomized Period
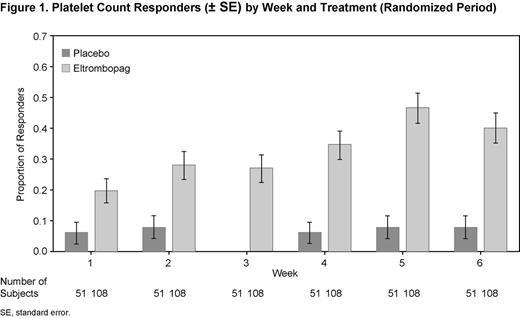
A higher proportion of EPAG versus PBO subjects (62% vs 24%; P < 0.001) achieved a response with platelet counts ≥50 Gi/L at least once between weeks 1–6 (Cohort 1, 64% vs 11%; Cohort 2, 64% vs 27%; Cohort 3, 54% vs 36%, respectively). At each week, a higher proportion of EPAG subjects had a response versus PBO (Fig. 1). A lower proportion of EPAG subjects (13%) received rescue treatment compared with PBO subjects (31%; P = 0.009). The odds of having World Health Organization (WHO) bleeding grades 1–4 (0.19; P = 0.011) and clinically significant (WHO grades 2–4) bleeding (0.29; P = 0.007) were lower for EPAG versus PBO subjects.
EPAG-Only Period
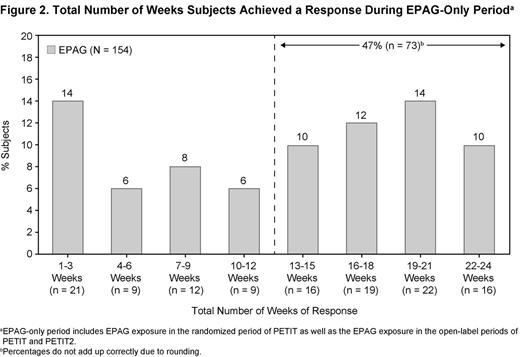
Sustained reduction or discontinuation of baseline ITP medications, primarily corticosteroids, was achieved by 50% of subjects; 81% of subjects had a platelet count response at least once; 52% (n = 80/154) had a platelet count response for ≥50% of assessments; and 38% (n = 58/154) responded for ≥75% of assessments. For >13 of 24 weeks, 47% of subjects achieved responses (Fig. 2). The median average daily dose for EPAG-exposed patients in Cohorts 1, 2, and 3 were 64.0 mg (0.93 mg/kg), 57.6 mg (1.50 mg/kg), and 37.0 mg (2.02 mg/kg), respectively.
AEs
Similar proportions of subjects in the EPAG and PBO groups reported an AE during the randomization period. The most common AEs (≥10% of subjects) were headache, upper respiratory tract infection, and nasopharyngitis in the EPAG group, and headache, epistaxis, and vomiting in the PBO group. Serious AEs (SAEs) were reported in 8% of EPAG subjects versus 12% of PBO subjects. No SAEs were reported by >1 subject in either treatment group except epistaxis, which was reported by 2 subjects in the PBO group. No SAEs were common to both treatment groups. In the randomized period, an ALT elevation of ³3 x ULN occurred in 5 (4.7%) subjects in the EPAG group and no subjects in the PBO group. In the OL period, there were an additional 7 subjects with ALT ³3 x ULN. All elevations resolved either while still on treatment or after discontinuation of study treatment. Overall, the hepatobiliary laboratory findings were mostly mild, reversible, and not accompanied by impaired liver function. Fewer EPAG than PBO subjects reported bleeding AEs (17% vs 36%, respectively). No thromboembolic events were reported. Cataract events were experienced by 2 subjects who received EPAG; both had used corticosteroids and 1 had pre-existing cataracts.
Conclusions: EPAG was safe and raised platelet counts in 62% of pediatric patients with persistent and chronic ITP during the randomized phase. Treatment with EPAG was well tolerated in both studies as evidenced by the low incidence of treatment discontinuations due to AEs.
Bussel:Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Honoraria; Novartis: Honoraria; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunomedics: Research Funding; IgG of America: Research Funding; GlaxoSmithKline: Equity Ownership, Honoraria, Research Funding; Genzyme: Research Funding; Eisai, Inc.: Research Funding; Cangene: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria; Amgen: Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Symphogen: Membership on an entity's Board of Directors or advisory committees; Sysmex: Research Funding. Off Label Use: Eltrombopag is a thrombopoietin receptor agonist approved for the treatment of thrombocytopenia in adults with chronic ITP. Use in children and adolescents will be discussed.. Grainger:GlaxoSmithKline: Honoraria; Baxter: Honoraria, Research Funding; Amgen: Honoraria. Pongtanakul:GlaxoSmithKline: Research Funding. Komvilaisak:GlaxoSmithKline: I am an investigator on this study. Other. Sosothikul:CSL Behring: Research Funding; GlaxoSmithKline: Research Funding. Drelichman:GlaxoSmithKline: I am investigator on this study. Other. David:GlaxoSmithKline: Research Funding. Marcello:GlaxoSmithKline: Employment. Iyengar:GlaxoSmithKline: Employment. Chan:GlaxoSmithKline: Employment. Chagin:GlaxoSmithKline: Employment. Theodore:GlaxoSmithKline: Employment, Equity Ownership. Bakshi:GlaxoSmithKline: Employment, Equity Ownership. Bailey:GlaxoSmithKline: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal