Abstract
Background: During hemostasis, platelets, as the first-responders of vascular injury, are subjected to a dynamic microenvironment. Biochemically, diverse and rapidly changing gradients of soluble proteins and agonists, such as von Willebrand factor, ADP, and thrombin, drive platelet adhesion and activation. Biophysically, the hemodynamic shear forces of the circulation can also activate platelets. As clot formation ensues, platelets then interact with polymerizing fibrin scaffolds, exposing platelets to a large range of mechanical microenvironments. While the underlying biochemical signaling pathways that govern the fibrinogen-¦ÁIIb¦Â3-mediated processes have been well characterized, if and how the mechanical cues in the fibrinogen microenvironment affect platelet physiology remain largely unknown. Therefore, a systematic approach to investigate how platelet activation is affected by the matrix mechanical properties, namely stiffness, could lead to profound insights into platelet physiology. To that end and to decouple the mechanical cues from biological and biochemical factors that may be involved (Lam WA et al, Mol Cancer 2010, 9: 35; Pathak A & Kumar S, P Natl Acad Sci, 109 (26): 10334-10339; Ulrich A et al, Cancer Res, 2009, 69 (10): 4167-4174) , we immobilized fibrinogen on polyacrylamide (PA) gels of varied stiffnesses (elastic moduli = 0.25-100 kPa, in the range of stiffnesses platelets may encounter in vivo), and investigated how platelets biologically respond to these differences in substrate stiffness.
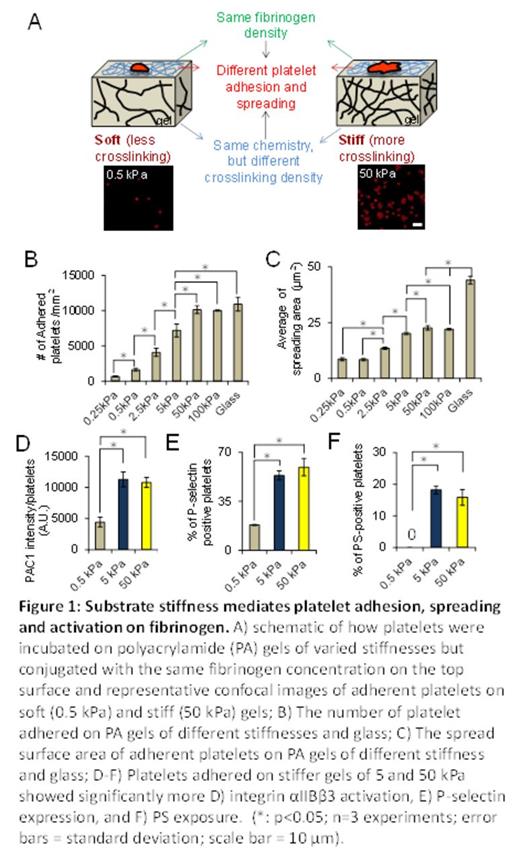
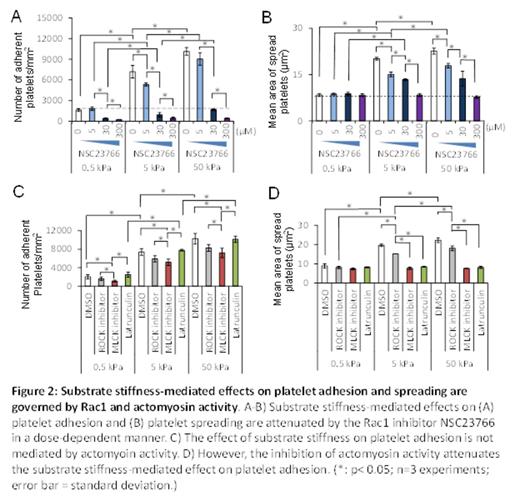
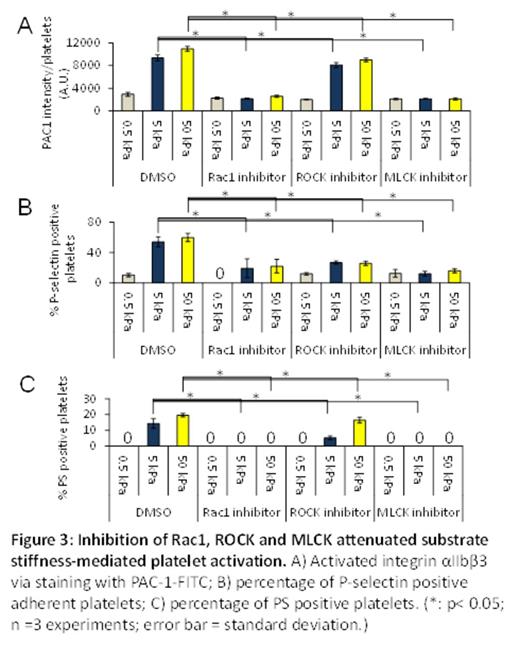
Results and Discussions: PA gels of different stiffnesses were covalently conjugated with constant fibrinogen concentrations, thus allowing us to only study the effect of mechanical properties of substrates on platelet physiology (Figure 1A). By simply varying the stiffness of the fibrinogen-bound PA gels, , we found that the number of adherent platelets on the PA gel surfaces after incubation increased with increasing substrate stiffness, reaching a plateau (~10,000 platelets/ mm2) on 50 kPa PA gels and stiffer (Fig. 1B). The average spread area of adherent platelets on gels of different stiffnesses also exhibited a similar trend as that of platelet adhesion and reached a similar plateau (~23 ¦Ìm2) on 50kPa gels and stiffer (Fig. 1C). More importantly, we also found that PA gels stiffer than 5 kPa caused more platelet activation, namely increased integrin ¦ÁIIb¦Â3 activation (Fig. 1D), P-selectin secretion (Fig. 1E), and phosphatidylserine (PS) exposure (Fig. 1F), as compared to softer gels. To investigate the underlying mechanisms that govern platelet mechanosensing during adhesion, spreading, and activation, we exposed platelets to a series of pharmacological cytoskeletal inhibitors. The substrate stiffness-mediated effect on platelet adhesion was eliminated by the Rac1 inhibitor NSC23766 in a dose-dependent manner (Fig. 2A-B). In addition, inhibition of actin polymerization with latrunculin and inhibition of myosin activity with the MLCK inhibitor ML-7 blocked the substrate stiffness-mediated effect on platelet spreading (Fig. 2C-D). In addition, inhibition of both Rac1 and actomyosin activity decreased platelet ¦ÁIIb¦Â3 activation, P-selectin secretion, and PS exposure on stiffer gels to the level similar to those on softer gels (Fig. 3A-C).
Conclusions: Our data demonstrate that platelets mechanosense the stiffness of the underlying clot substrate, and increasing substrate stiffness increases platelet adhesion and spreading. Importantly, adhesion on stiffer substrates leads to higher levels of platelet activation. Mechanistically, we determined that Rac1, actin, and myosin activity mediate this process. This newfound capability of how platelets adjust their degree of activation based on the mechanical properties of their microenvironment provides new insight into how clots are formed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal