Abstract
Introduction:Children with sickle cell disease (SCD) and abnormal cerebral blood flow often receive cyclic blood transfusions (CTx) to reduce risk of stroke. While effective for stroke prevention, CTx causes systemic iron accumulation that may damage multiple organs (Harmatz P., Blood 2000). Iron toxicity from CTx is poorly described for the brain, in comparison to the liver and heart. Quantifying iron in the subcortical structures and the choroid plexus is of renewed clinical importance, due to recent findings that iron accumulation in these regions may predict CNS injury in neurodegenerative disorders. In this study, a new imaging protocol is applied to quantify iron levels and measure structural volumes for the subcortical structures and choroid plexus of children with SCD on CTx, using healthy children for comparison.
Methods:SCD patients were eligible if at least 12 consecutive months of CTx were received for abnormal transcranial doppler. Patients with history of CVA were excluded, due to potential confounding effects of prior ischemic damage.Healthysiblings and relatives were used as ethnic- and age-matched controls.MRI scans of the liver and brain, using a Siemens 3T Trio scanner, was performed without sedation for all subjects. A modified pulse sequence (R2*) for the 3T scanner was used to determine liver iron concentration. T1-weighted imaging sequence with MPRAGE was used for anatomical identification and volume measurements of brain regions (Kirk G., Cerebral Cortex 2009). A 3D multi-echo gradient-echo sequence (FOV=256x192mm, Matrix=256x192, Slice thickness = 2mm, 72 slices, TR = 50 ms, first TE = 3.7ms, 12 echoes, echo spacing = 3.8ms) was used for the quantitative susceptibility mapping (QSM), a MRI method that is sensitive to tissue iron (Shmueli K., Magn Reson Med 2009). Iron levels were generated with a post-processing protocol developed for this application (modification of Qiu D., AJNR 2014). To compare iron levels between the two groups a general linear model (GLM) was used that incorporated the ages of the subjects into the comparison. For the patients, an additional GLM analysis was performed to determine if iron level when in each region when corrected for age was associated duration of transfusions, liver R2* and average serum ferritins.
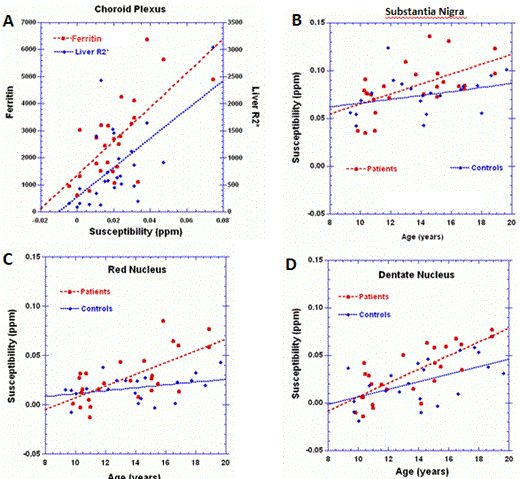
Results:MRI scans were evaluable for 25 SCD patients on CTx and 23 controls. There were no significant differences between patients and healthy controls with respect to age, sex or ethnicity. The average duration of CTx was 6.0 (1.5-12.6) yrs and the average serum ferritin and liver iron R2* were 2540 ng/ml and 819 Hz (~14.8mg/g dry weight), respectively. As anticipated, iron level in the choroid plexus was significantly elevated in patients compared to controls (p=1.4x10-7), and levels correlated well with serum ferritin (p=0.0003) and liver R2* (p=0.001) (panel a). While there were no significant differences detected between the groups with respect to iron levels in the subcortical structures, there was a trend to increasing differences between the groups with age in the Substanita Nigra, Red Nucleus and Dentate Nucleus (panels b,c,d). In the GLM analysis, age was significantly associated with iron levels in the subcortical structures, not in the choroid plexus. Although iron levels in some of the subcortical structures were associated with duration of CTx, ferritin and liver R2*, the significance was predominately lost when corrected for age. These observations suggest additional studies with older subjects may reveal increased differences. Volumetric analysis was performed to assess potential injury to deep gray matter. The measured volumes of the subcortical structures were not statistically different between the groups, with a noted exception of the caudate nucleus (p=0.021), a region of known predisposition to injury in SCD (Steen R., Ann Neurol 1999).
Conclusions: The MRI protocol successfully quantified iron in brain structures, associated with neurodegenerative disorders. Iron accumulation in the choroid plexus was significant in SCD patients on CTx, and correlated with other markers of iron overload. While iron was detectable in subcortical structures, the levels were not significant different from controls after adjusting for age. These finding should be helpful in planning for a longitudinal study that includes a broader age range.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal