Abstract
Introduction: Sickle cell disease (SCD) is a common inherited blood disorder caused by sickle hemoglobin (HbS) which, unlike normal adult hemoglobin (HbA), becomes insoluble and polymerizes under hypoxic conditions. Patients with SCD experience chronic hemolytic anemia, episodic pain crises and abnormal blood flow to critical organs that cumulatively result in significant illness and shortened lifespans for many. The severity of SCD varies significantly between patients, but for individuals the rate of adverse events is strongly correlated with intraerythrocytic concentration of HbS (%HbS). High per test costs and long turnaround times make conventional laboratory methods (e.g. Hb electrophoresis, HPLC, IEF) impractical for quantifying %HbS in real-time (e.g. during transfusion therapy). The objective of this study was to demonstrate that %HbS in blood could be quantified using our recently developed rapid, low-cost paper-based SCD assay [1].
Methods: Blood samples were obtained from SCD and sickle cell trait (SCT) patients at the Texas Children’s Hematology Center (Houston, TX). To perform the SCD assay a 20μL droplet of blood mixed with Hb solubility buffer (1:10 by volume) was dropped on chromatography paper. The resulting blood stain was digitized with a flatbed scanner (Canon USA Inc, Melville, NY) and analyzed using a custom image analysis code (The MathWorks Inc, Natick, MA). Conventional Hb electrophoresis was performed with the semi-automated Sebia Hydrasys 2 Scan system (Sebia Inc, Norcross, GA).
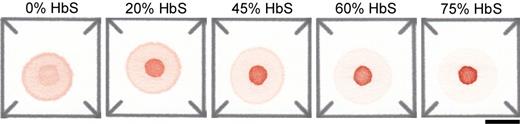
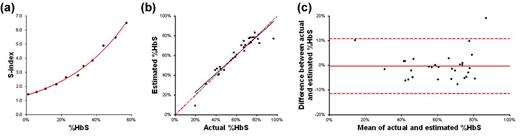
Results: The difference in transport of Hb through the paper produced a blood stain with two parts: the area of the initial drop where polymerized HbS is retained (center spot) and the area where soluble Hb is wicked laterally (peripheral ring). The relative color intensity of the center spot and peripheral ring is related to the blood sample %HbS (Fig. 1). The image analysis code automatically isolates and calculates the ratio of the average color intensities of each area (S-index). A series of reconstituted blood samples with artificially adjusted %HbS from 0 to 60% was used to calibrate the assay so that %HbS could be estimated based on blood stain color intensities (Fig. 2a). The values of %HbS estimated for patient samples using our paper-based SCD assay and actual values measured using conventional Hb electrophoresis were highly correlated with R2 = 0.898 (Fig. 2b). The estimated and actual %HbS values also showed strong agreement with the standard deviation of the difference between the two measurements = 5.5 %HbS (Fig. 2c). The majority of the differences between actual and estimated %HbS (96.67%) are within 2 standard deviations of the mean of the differences. The assay could be performed in under 35 minutes and multiple assays could be performed and analyzed in parallel. The cost of consumable materials and reagents for the paper-based SCD assay is less than $0.03.
Conclusions: This study demonstrates the feasibility of using our recently developed paper-based assay to quantify %HbS in blood samples in real-time. The ability to rapidly, inexpensively measure %HbS will be particularly useful for monitoring the effectiveness of chronic transfusion or hydroxyurea therapy for long-term control of HbS content in blood of SCD patients. The ability to measure %HbS in real-time could also potentially facilitate more aggressive prophylactic therapy to intervene rapidly and significantly reduce the rate of life-threatening complications in SCD patients, including stroke.
Acknowledgments: This work was supported in part by a 2012 NIH Director's Transformative Research Award (NHLBI R01HL117329, PI: SSS).
References:
[1] Yang X, et al. Lab Chip, 2013, 13, 1464-1467.
Piety:Tulane University: PCT/US2012/064856 Patents & Royalties. Yang:Tulane University: PCT/US2012/064856 Patents & Royalties. Shevkoplyas:Tulane University: PCT/US2012/064856 Patents & Royalties; Halcyon Biomedical Incorporated: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal