Abstract
Autoimmune hemolytic anemia (AIHA) is a greatly heterogeneous condition both in terms of clinical presentation and response to treatment, usually classified as warm (WAIHA), cold (CAD), mixed, and atypical forms. The aim of this study was to identify predictors of outcome and response to therapy considering in particular the serological characteristics and the severity of anemia at onset. We evaluatedretrospectively 307 patients (112 M and 195 F, median age at diagnosis 63, range 1-97), diagnosed between 1978 and 2013 and followed-up for a median of 33 months (range 12-372); 60% of cases were WAIHA, 27% CAD, 8% mixed, and 5% atypical (14 DAT- and 1 DAT+ for IgA only). Hemoglobin values were lower in mixed (median 5.8, range 2-10.7 g/dL) atypical (6.2, 3-9), and in IgG+C3 DAT+ WAIHA (6.9, 2.9-11.5). Twenty-one subjects were diagnosed with Evans’ syndrome, the majority of them WAIHA, with a severe onset. Considering anemia at onset, 27% of cases had Hb levels <6, 36% Hb 6.1-8, 24% Hb 8.1-10, and 13% Hb>10 g/dL; the most severe cases were mainly mixed and atypical forms (P=0.0001).
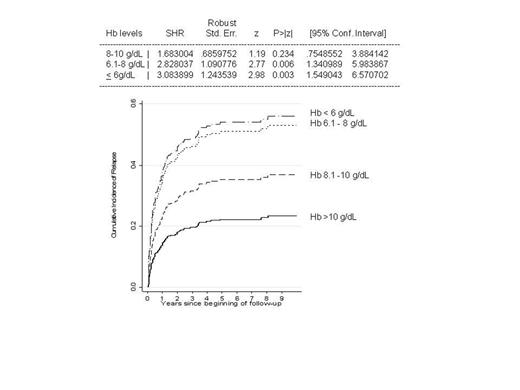
Regarding therapy, 47% of cases were treated with one therapy line only, 26% with two, 13% with three, and 4% with four or more lines. Sixty % of WAIHA received first line steroid therapy only, 20 CAD required no treatment, and patients with IgG+C DAT+ WAIHA, mixed, and atypical forms were more frequently treated with 2 or more therapy lines (P<0.0001); the gender- and age-adjusted cumulative incidence of relapse was significantly increased in more severe cases by Fine and Gray model (Figure). Response to steroids was observed in ~75% of cases, with lower rates in CAD and generally observed at high steroid dosages. Splenectomy (32 cases, mostly WAIHA or severe forms) had a response rate of 75%, but was ineffective in 2/3 CAD; the relapse rate was 8/24 (33%) after a median of 41 months. Regarding immunosuppressants (31 cases azathioprine, 40 cyclophosphamide, and 12 cyclosporine) the OR was 50-70% (PR 20-40), irrespective of serological type and severity of anemia, although the simultaneous administration of steroid in most cases may weaken these results; the relapse rate was 8/60 (13%) after a median of 11 months. Rituximab (55 cases at conventional, and in 19 at low doses (LD) of 100 mg /weekly x 4) had an 80% OR (35% PR). Predictors of response to LD were WAIHA, younger age, and shorter interval between diagnosis and rituximab therapy; at variance, OR to conventional doses occurred irrespectively of age, serological type, clinical severity at onset, and disease duration. The relapse rate was 5% (2/42, of whom 1 CAD) for standard and 38% (6/16, of whom 5 CAD) for LD, and relapses occurred mostly within the first year after treatment.
As regards complications, infections occurred in 26 cases (10 grade 3, 11 grade 4, and 5 grade 5), irrespective of serological AIHA type and severity at onset, and of the number of therapy lines; on the contrary, they were observed more frequently in splenectomized cases. Acute renal failure was recorded in 6 cases and was not associated with AIHA clinical or serological characteristics. A thrombotic event was recorded in 11% and was associated with severe onset, higher median LDH levels, and previous splenectomy.
At the time of the analysis 63 cases (21%) have died, of whom 11 because of AIHA (3.6%); death was not associated with the severity of anemia at onset, nor with the serological type of AIHA; at variance, it was associated with infections (HR 11.47, 95% CI 3.43-38.4, p=0.0004), acute renal failure (HR 17.99, 95% CI 4.73-68.40, p=0.001), Evans’ syndrome (HR 6.8, 95% CI 1.99-23.63, P=0.0074), previous splenectomy (HR 3.21, 95% CI 0.92-11.25), and multi-treatment (4 or more lines of therapy; HR 9.1, 95% CI 2.41-34.36, p=0.0076). Death was not associated with thrombotic events, nor with the type of treatment, in particular immunosuppressants or rituximab.
In conclusion, we showed that AIHA cases with a severe onset, mostly mixed and atypical forms, are frequently refractory to different therapies. Although obtained retrospectively, our results suggest to put forward rituximab among second line options, given its efficacy and safety. In addition, standard rituximab doses should be preferred in CAD, whereas lower doses may be equally effective in WAIHA and mixed forms. Finally, we suggest to defer splenectomy after rituximab, given the increased risk of thromboembolism, infections and fatal outcome in splenectomized patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal