Abstract
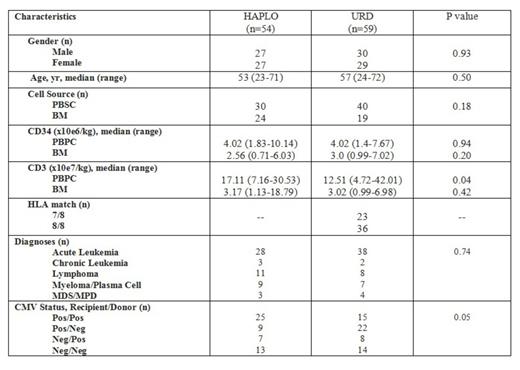
Haploidentical (HAPLO) and unrelated donors (URD) are established cell sources for patients (pts) who lack HLA-matched siblings. Limited data are available comparing transplant (TP) outcomes using cells from HAPLO vs URD donors. We performed a retrospective analysis of sequential pts (n=54) undergoing HAPLO transplants with post-transplant cyclophosphamide (Cy). A control group of HLA 7/8 or 8/8 matched URD recipients (n=59) matched by diagnosis, transplant date, and cell source (PBSC or marrow) was identified (Table). Most HAPLO pts received cycles of pre-TP fludarabine (Flu) for lymphodepletion to achieve CD4 <0.2x109/l. HAPLO pts received Flu 30 mg/m2 qd x 5 with Cy 14.5 mg/kg qd x 2, followed by TBI, 200-400 cGy in 1 or 2 fractions (physician discretion). PB (n= 30) or BM (n= 24) was infused from 1st or 2nddegree related HAPLO donors. Post-transplant immunosuppression was Cy 50-60 mg/kg on days +3 and +4, followed by tacrolimus (tacro) and mycophenolate (MMF) starting on day +5. Pts received filgrastim daily starting at day +5. URD recipients were conditioned with a variety of standard myeloablative (n=23) or reduced intensity (n=36) regimens, and received PB (n= 40) or BM (n= 19), with daily filgrastim starting on day +9. GvHD prophylaxis was tacro and short-course methotrexate (n= 53) or tacro/MMF (n=6). CD3+ and CD15+ cell chimerisms were assessed at 4 week intervals commencing at day +28. Bone marrow staging and CD34+ cell chimerism were assessed at day +84. GvHD was staged per standard guidelines. Analysis of categorical variables was performed using the Fisher's exact test. Non-parametric analysis of median values was used for continuous variables. Survival was assessed using the log-rank test. Informed consent for analysis of transplant outcome data was obtained before transplantation for all patients and donors.
Engraftment kinetics in the HAPLO cohort was slightly slower compared to the URD cohort, ANC >0.5x109/l at a median of 16 days (range: 13-45) vs 13 days (range: 9-25, p<0.001); PLT >20x109/l median 24 days (range: 13-109) vs 15 days (range: 8-216, p= 0.02). ANC and PLT recoveries were faster for URD PB compared to BM recipients. However, engraftment kinetics did not differ for HAPLO PB or BM recipients, (ANC median days 16.5 vs. 16, p=0.38; PLT median 24 vs 26 days p=0.80). HAPLO donor CD3 engraftment was robust, with 44 of 48 (92%) HAPLO compared to 37 of 52 (71%) URD recipients achieving >95% CD3+ chimerism at day +28 (p=0.01). Engraftment failure defined by failure to achieve ANC recovery and day +28 donor CD3 chimerism >5% was reported in 4 (7.4%) HAPLO and 2 (3%) URD recipients (p=0.42).
The cumulative incidence of grade II-IV aGVHD by day 100 was similar for both groups (61% vs 47%, p=0.19). The cumulative incidences of cGVHD in the HAPLO vs URD cohorts were comparable (39% vs 36%, p=0.84) and the incidence of moderate or severe cGVHD was 15% and 22%, respectively (p=0.34). At time of analysis, relapse occurred for 37% HAPLO pts (n=20) compared to 33% (n=20) in the URD group (p=0.84). 23 pts died (43%) in the HAPLO group: 12 from relapse, 5 from infection, 6 from other causes. In the URD group, 23 (39%) died: 13 from relapse, 3 from infection, 7 from other causes. The one-yr survival probabilities were 56% HAPLO and 66% URD, with median OS probabilities 18 mo and 22 mo (p=0.85). OS was lower for recipients of 7/8 vs 8/8 matched URD grafts, but the difference was not significant (not shown).
These results show similar outcomes after HAPLO and URD transplant for engraftment, GvHD, relapse and OS. Almost all HAPLO pts achieved full donor CD3+ chimerism by day +28. In conclusion, HAPLO transplant with post transplant Cy is an option for pts lacking HLA matched siblings; prospective studies are needed to validate these findings.
;
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal