Abstract
Background: Most patients with acute myeloid leukemia (AML) die from relapsed disease, including those who attain complete remission (CR). Post-remission treatment designed to kill chemoresistant leukemia stem cells (LSC) and eradicate minimal residual disease (MRD) could delay or prevent relapse. The Interleukin-3 Receptor alpha chain (IL3Rα/CD123) is overexpressed on LSC and AML blasts compared to normal hematopoietic cells. CSL362 is a fully humanized anti-CD123 monoclonal antibody, engineered for greater antibody-dependent cell-mediated cytotoxicity (ADCC) by high affinity for NK cell CD16. CSL362 has potent activity against AML cells in vitro and in mouse xenograft models. In this Phase 1 study we evaluated the safety, PK and immunogenicity of repeat doses of CSL362 as post-remission treatment for AML patients. Exploratory endpoints include effects on CD123+ normal blood cells (basophils and pDC), NK cells, levels of serum cytokines, CSL362 binding to CD123 on monocytes, and MRD.
Methods: Eligibility criteria include CD123+ AML in 1st or 2nd CR /CRp, adverse risk factors (disease history, cytogenetics, molecular mutations or MRD), and no plan for allogeneic stem cell transplantation. Intravenous CSL362 is given over 3 hours every 14 days x 6 doses. Final evaluation for safety is at week 16 and for remission status is at week 24. Sequential cohorts of 3 - 6 pts have been treated at 5 dose levels, from 0.3 to 9.0 mg/kg. Serum is assayed centrally for cytokines, PK and immunogenicity. Standardized whole blood biomarker assays are performed at each site by flow cytometry, and analyzed centrally. Bone marrow is sampled at baseline, weeks 5 and 12.
Results: To date, 25 pts (16M, 9F) median age 66 yr (range 32-83 yr) have received 118 infusions of CSL362; 15 pts received all 6 doses, 6 pts relapsed on study, 1 pt was withdrawn for an SAE (infusion reaction); 3 pts are ongoing. Adverse events related to CSL362 in ≥ 10% of pts (CTCAE V4 grades) were infusion reactions (12 pts; 15 grade 1-2, 2 grade 3 events), increased C-reactive protein (3 pts; 4 grade 2 events), hypertension (3 pts; 6 grade 1-2, 2 grade 3 events), and hypotension (3 pts; 2 grade 2, 1 grade 3 events). There have been 3 dose limiting toxicities (1 hypertension, 2 infusion reactions), and 3 related SAEs (2 infusion reactions, 1 transient delirium). Infusion reactions have been manageable with supportive care. A protocol amendment to allow hydrocortisone premedication has been effective to prevent or reduce the severity of reactions. At follow-up ≥ 6 months from first dose, 10 of 20 evaluable pts maintained CR. Median duration of CR from start of CR and ongoing at last follow-up was 34+ weeks [range 26 – 52+ weeks]. Of 6 evaluable pts who were MRD positive at baseline, 3 converted to negative on study.
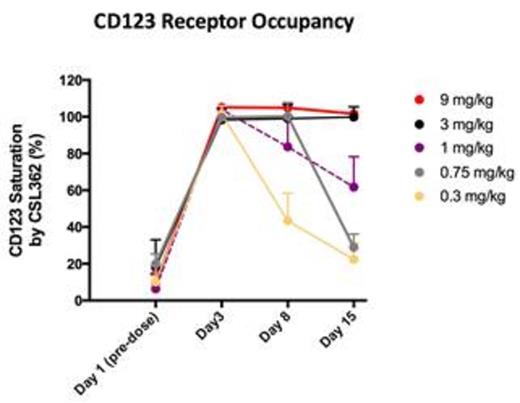
Biomarker assays show rapid, complete in vivo depletion of basophils and pDC (cells that have high expression of CD123) at all dose levels, ≤ 6 hours post-dose. Depletion is sustained for ≥ 15 days at doses ≥ 3.0 mg/kg. NK cells (which do not express CD123) in blood fall transiently then recover from day 3 post-dose to ≥ baseline by day 8. CSL362 saturation of CD123 on monocytes (cells that have low CD123 expression) in vivo occurs rapidly post-dose at all dose levels and is sustained ≥ 15 days at dose levels ≥ 3.0 mg/kg. Serum CSL362 concentration demonstrated a nonlinear PK profile with slower clearance and longer half-lives at higher doses. PK profiles at doses above 3 mg/kg seem to be dose proportional. The half-life for the two higher dose levels is 4-5 days. Cytokine assays showed increased levels of GM-CSF, IFN-γ, IL-1β, IL-6, IL-8, TNF-α, IL-15 and IL-1RA after the 1st dose, returning to baseline by day 8. Levels after dose 6 were lower than after the dose 1. Low level anti-CSL362 antibody titers were detected coincident with trough concentrations of CSL362 in 6 of 18 pts tested thus far, with no effects on PK or association with adverse events.
Conclusion: CSL362 is safe and well tolerated in AML pts with CR/CRp and high risk of relapse. Potent, targeted ADCC of normal cells (basophils and pDC) that highly express CD123 is evident at all dose levels tested, with durable depletion at ≥ 3.0 mg/kg. At dose levels ≥ 3.0 mg/kg, saturation of CD123 on marker monocytes is durable for the inter-dose interval. These data indicate it is likely that dose levels ≥ 3.0 mg/kg are required to maximize ADCC against residual AML. A phase 2 study of CSL362 is planned.
Roboz:Glaxo SmithKline: Consultancy; Celgene: Consultancy; Agios: Consultancy; Novartis: Consultancy; Astra Zeneca: Consultancy; Sunesis: Consultancy; Teva Oncology: Consultancy; Astex: Consultancy. Busfield:CSL Limited: Employment. Barnden:CSL Limited: Employment. Sedgmen:CSL Limited: Employment. Ghosh:CSL Limited: Employment. Hosback:CSL Limited: Employment. Davis:CSL Limited: Employment. Dyson:CSL Limited: Employment. Dasen:CSL Behring: Employment. DeWitte:CSL Behring: Employment, Equity Ownership. Bensen-Kennedy:CSL Behring: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal