Abstract
Background:
Success of single unit UD-CBSCT has been limited by graft failure, delayed engraftment and high early mortality. Preparative regimen of Busulfan with TF and horse ATG has reduced early mortality and improved engraftment in single unit UD-CBSCT in adults (Sanz. BMT 2012: 47; 1287-1293). M with TF and r-ATG has previously been used as a preparative regimen in double expanded UD-CBSCT in adults (De-Lima. NEJM 2012: 367; 2305-2315). Outcomes of this regimen will be of interest in single unit UD-CBSCT.
Methods:
We retrospectively analyzed the outcomes of all patients that underwent UD-CBSCT from single unit using MTF-rATG conditioning at UMass Memorial Medical Center.
Results:
Twenty five underwent single unit UD-CBSCT from January 2009 to June 2014. There were 15 males and 10 females. The median age was 63 years (range, 28 -81) and median weight was 68.9 kg (range, 58.9 - 119). Nine patients (36%) were over age 65 years. Diagnosis was AML/MDS (n= 18), CLL (n= 2) and others (n=5)- ALL/Aplastic Anemia/MM/NHL/Blastic NK cell malignancy. Disease status CR1 (n=11), CR2 (n=1) and persistent disease (n=13). Four patients had undergone a prior autologous SCT.
Preparative regimen consisted of T (5-10 mg/kg) day -7, F (30 mg/m2) from day -6 to -2, M (100-140 mg/m2) day -1 and r-ATG (3 mg/kg). Graft versus host disease (gvhd) prophylaxis consisted of mycophenolate mofetil (day -1 to 56) and tacrolimus (level 5-15 ng/ml) starting day -1. Tacrolimus was replaced in 2 patients: Sirolimus (n=1) and Cyclosporine (n=1). HLA matching was 6/6 (n=1), 5/6 (n=1) and 4/6 (n=23). The median total nucleated cell (TNC) dose based on pre-cryopreserved sample was 3x10e7/kg (range, 1.9 - 5.3). The median TNC and CD34 cells infused was 2.5x10e7/kg (range, 1.3-4.2) and 1.13x10e5/kg (range, 0.16-3.05) respectively.
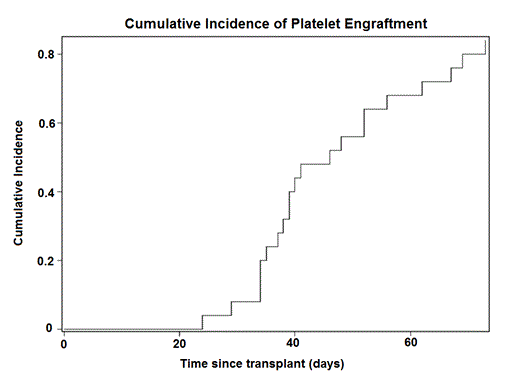
Day 100 mortality was 16% (CI,6-37%). Cumulative incidence of neutrophil engraftment (NE) was 92% (CI, 62- 99%) and that of platelet engraftment (PE) was 84% (CI, 57- 95%) Figure 1 & 2.The median time of NE was 19 days (range, 13-37) and that of PE was 40 days (range, 24-73). All 23 patients surviving beyond day 10 engrafted their neutrophils. “Transplant Success" defined by a composite end point (NE by day 26, PE by day 60 and survival at day 100) was 60% (CI,38% -77%). The incidence of acute gvhd (I &II only) was 18% (CI,6-41%) and that of chronic gvhd was 25% (CI10-49%). 9 patients are currently alive at a median followup of 932 days (range, 97-1875 ) .All survivors are gvhd free and off immune suppression.The 2 year overall survival (OS) based on Kaplan Meier estimate is 36 %(CI,18-55%). Figure 3
Discussion:
Preparative regimen of MFT-rATG results in excellent engraftment, low early mortality and acceptable “Transplant Success” in single unit UD-CBSCT in older adults. Our outcomes in high risk older adults compare favorably to other studies using “double cord” or “cord blood expansion” strategies.
Nath:Celgene: Consultancy, Honoraria. Off Label Use: Thiotepa,Melphalan,rabbit ATG,Fludarabine, mycophenolate mofetil All these have been used in preparative regimens and gvhd prophylaxis for allogeneic stem cell transplantation. Cerny:Incyte: Consultancy, Honoraria; Ariad Pharmaceuticals: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal