Abstract
Introduction: Autoimmune hemolysis (AH) & immune thrombocytopenic purpura (ITP) are recognized complications of CBT. However, the incidence, severity, treatment response, & prognosis of these autoimmune cytopenias are not established.
Methods: We evaluated AH/ITP after CBT in a landmark analysis of 152 patients who received double-unit grafts, had sustained donor engraftment, & were disease-free at 100 days post-transplant. This landmark was chosen as no patient has developed AH/ITP prior to day 100. CBT recipients (median 38 years, range 0.9-70) were transplanted for hematologic malignancies with myeloablative (MA) or non-myeloablative (NMA) conditioning and calcineurin-inhibitor (CNI)/mycophenolate mofetil immunosuppression.
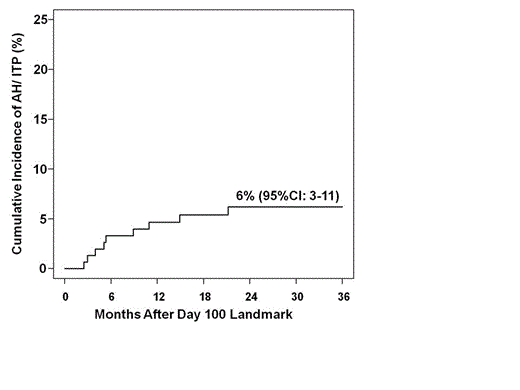
Results: With a median follow-up of survivors in this cohort of 50.6 months (range 7.6-105.4) post-CBT, 9 patients [median age 42 years (range 2-54), 5 MA & 4 NMA conditioning] have developed autoimmune cytopenias (7 AH, 1 ITP, 1 both). All AH patients were IgG Direct Antiglobulin Test (Coombs) positive and ITP diagnosis was made by standard criteria. The cumulative incidence of AH/ITP is 6% (95%CI:3-11) at 3-years after the day 100 landmark with a median onset of 8.6 months (range 5.8-24.5) post-CBT (Figure). Six patients presented with severe disease (Hb <6 gm/dl &/or plts <20) requiring immediate aggressive supportive care. The lowest counts (Hb 2.6-6.8 & plts 0-4) were observed a median of 1 day (range 0-94) after diagnosis. Six patients had grade II-IV acute GVHD (onset 17-175 days post-CBT, all prior to development of AH/ITP), and all 9 cases developed in the context of immunosuppression taper. No relationship was observed according to age, diagnosis (acute leukemia vs lymphoma), preparative regimen intensity (MA vs NMA), or recipient CMV serostatus. Treatment during the first week included IVIg/corticosteroids/rituximab in 3 patients or a variety of approaches (1 increased CNI dose, 2 IVIg only, 2 rituximab only, 1 corticosteroids/IVIg). Overall, all 9 patients received rituximab starting 2-18 days (4-6 doses) with initial treatment. Early rituximab at <7 days from diagnosis reduced time to complete response (CR, Hb >8 &/or plts >100): median 13 days (7-49 days) in 4 early rituximab patients vs 58 days (19-98 days) in 5 without early rituximab. Moreover, an initial IVIg/corticosteroids/rituximab combination was best (CR 7-13 days). Four patients flared after CR at 28-393 days & all patients achieved CR with further therapy. Three patients underwent splenectomy (2 with initial therapy & 1 with flare). Eight/9 AH/ITP patients are alive & disease-free (one patient died of GVHD in remission from AH). Seven/8 surviving patients are in CR from AH/ITP (median 30 months after AH/ITP diagnosis, range 9-102) & 1 has recurrent AH requiring therapy. Treatment was well tolerated although 4 patients required intermittent IVIg & 4 had transient neutropenia. Conclusions: AH/ITP occurs infrequently after CBT but is associated with sudden onset and may be life-threatening. The onset during immunosuppressant taper suggests the mechanism may be due to transient immune dysregulation at this time-point, and investigation of whether AH/ITP can be predicted from analysis of B-cell immune reconstitution is underway. IVIg/corticosteroid/rituximab combination is appropriate initial therapy in severe disease & our data suggests this is the most effective although the role of IVIg is unclear. Earlier treatment with Rituximab may reduce corticosteroid exposure & avoid early splenectomy. While the optimal treatment regimen is not established, our series suggests rituximab is essential to achieve & maintain CR & has an excellent safety profile. Finally, survival was high in AH/ITP patients with no deaths from this complication although this was likely contingent on aggressive supportive care in these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal