Abstract
Introduction:
High resolution human leukocyte antigen (HLA) matching is regarded as a prerequisite for the clinical success of allogeneic haematopoietic cell transplantation (HCT). However, studies have reported immunogenetic factors other than HLA also plays an important role in preventing and controlling post HCT complications, in particular graft-versus-host disease (GVHD) and infections. Cytokines and cytokine receptors, which are the key regulators of the immune surveillance against infections and/or allo-immune responses, are important candidates. The genetic control of cytokine production is evidenced by polymorphisms in cytokine gene regulatory regions resulting in low, moderate, or high cytokine production. Here, we evaluated the prognostic significance of cytokine genetic variants known to impact cytokine production on different outcomes of allogeneic HCT.
Materials and Methods:
A total of 793 subjects were accrued, including 360 adult allo-HCT donors (discovery cohort; n=240, and validation cohort; n=120), 382 HCT allo- recipients and 50 healthy individuals. All subjects of the discovery cohort and healthy individuals were analyzed for 22 single nucleotide variants located in the regulatory and/or exonic regions of 13 cytokine or cytokine receptor gene using sequence-specific primer based assay. All subjects of the validation cohort were genotyped SNPs located in the IL10 and IL1R gene promoter region by direct sequencing. . CMV specific immune response was assessed by stimulating PBMNCs from healthy individuals with CMV lysate and CMV peptide-pp65 followed by enumeration of IFN-g producing and CD107a expressing (degranulating) MNCs, T cells and their subsets.
Results:
Cytokine gene variants of donors and not that of recipients appear to influence post HCT complications. Two of these variant system- SNPs leading to low production of IL10 and high production of IL1R showed strong correlation with GVHD and posttransplant CMV infections respectively.
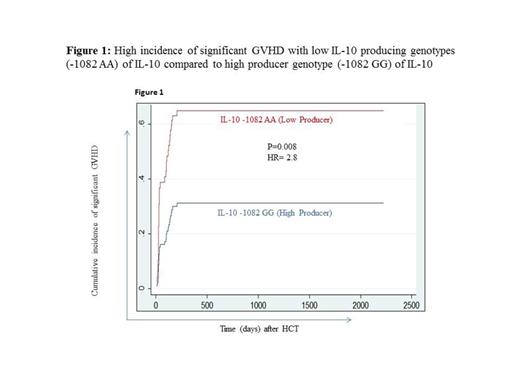
Allo-HCT recipients who have received graft from donors carrying low IL-10 producing gene variants (AA) at IL-10 -1082G/A and (ATA/ATA at IL-10 -1082G/A, -819C/T and -592C/A) have high incidence of significant GVHD (defined as grade II-IV acute GVHD and/or chronic GVHD requiring systemic immunotherapy) (P=0.008, HR= 2.8, Figure 1; and P=0.03, HR= 1.8 respectively) compared to recipients who have received graft from donors carrying high producer IL-10 gene variants.
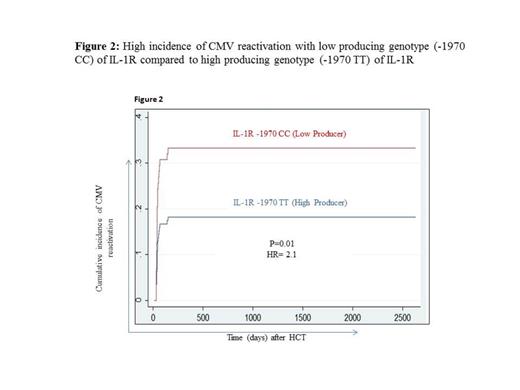
Allo-HCT recipients who have received graft from donors carrying low IL-1R producing genotype (-1970 CC) showed high incidence of CMV reactivation (p=0.01; HR=2.1, Figure 2) in comparison to the HCT recipients receiving grafts from donors carrying high IL1-R producing genotype (-1970 GG). Further, the in-vitro culture analysis in healthy individuals showed that IL1R+ cells have 3-5 fold stronger anti-CMV immune responses (IFNg+ and/or CD107a+ cells) in comparison to IL1R- cells.
Conclusions:
Genetic predisposition to low IL-10 production is a strong predictor of GVHD while that tohigh production of IL-1R confers strong protection against CMV reactivation after allogeneic HCT. Thefindings implicate the importance of prospective assessment of cytokine gene variants to improve allogeneic HCT donor selection.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal