Key Points
C-terminal domain determines myosin II localization to the MK contractile ring and the specific role of NMII-B in MK polyploidization.
Abstract
Endomitosis is a unique megakaryocyte (MK) differentiation process that is the consequence of a late cytokinesis failure associated with a contractile ring defect. Evidence from in vitro studies has revealed the distinct roles of 2 nonmuscle myosin IIs (NMIIs) on MK endomitosis: only NMII-B (MYH10), but not NMII-A (MYH9), is localized in the MK contractile ring and implicated in mitosis/endomitosis transition. Here, we studied 2 transgenic mouse models in which nonmuscle myosin heavy chain (NMHC) II-A was genetically replaced either by II-B or by a chimeric NMHCII that combined the head domain of II-A with the rod and tail domains of II-B. This study provides in vivo evidence on the specific role of NMII-B on MK polyploidization. It demonstrates that the carboxyl-terminal domain of the heavy chains determines myosin II localization to the MK contractile ring and is responsible for the specific role of NMII-B in MK polyploidization.
Introduction
Megakaryocytes (MKs) are the naturally polyploid hematopoietic cells that give rise to platelets.1-3 Polyploidization occurs by endomitosis, a process characterized by a cytokinesis failure related to a defect both in Rho activation and myosin II accumulation in the contractile ring.4-8 Our previous studies8 have revealed that nonmuscle myosin II (NMII) B (MYH10) is the only NMII present in the contractile ring during MK mitosis and endomitosis, but its expression is silenced during MK maturation both in human and mice megakaryopoiesis. This induces the switch from mitosis to endomitosis and allows MK polyploidization. Contrary to NMII-C, which is undetectable, NMII-A is well expressed during MK differentiation but is not recruited in the contractile ring whether MKs are in mitosis or endomitosis.6,8 These results revealed that NMII-B and NMII-A must display distinct functions during MK polyploidization and cytokinesis. However, these results were obtained with in vitro studies and need to be confirmed in in vivo models. Furthermore, some myosin II functions are determined by their distinct cellular localizations, which are controlled by the carboxyl (C)–terminal domain, whereas others are reliant on the motor activity of their amino (N)–terminal domain.9-13 Here, we have studied 2 mouse lines, in which the murine NMII-A (Myh9) heavy chain first coding exon was disrupted either by a complementary DNA (cDNA) encoding a green fluorescent protein (GFP) human NMII-B (Ab*/Ab* mice) or by a cDNA encoding a chimeric GFP human nonmuscle myosin heavy chain (hNMHC) II-AB (the N-terminal domain of NMII-A fused to the C-terminal II-B domain, Aab/Aab mice).10 Results obtained with these 2 models provide in vivo evidence for the specific role of NMII-B on MK polyploidization. They reveal also the important role of the C-terminal domain of NMII, which determines the specific NMII-B localization in the MK contractile ring.
Study design
Mice
Ab*/Ab* and Aab/Aab mouse lines were generated in the Adelstein’s laboratory.10 The detail of mice and genotyping are presented in supplemental Figure 1 (available on the Blood Web site).
In vitro cultures and sorting murine MK
Lin− cells were isolated by using the Lineage Cell Depletion Kit (Miltenyi Biotec, CA) and cultured in serum-free medium as previously reported8 and as described in supplemental Methods.
Lin−Sca-1+cKit+ (LSK) purification
Lin− bone marrow cells were isolated and labeled with PE-anti-Sca-1 (BD Pharmingen) and APC-anti-cKit (eBioscience) antibodies. LSK cells were sorted by an Influx flow cytometer.
In vivo and in vitro MK ploidy analysis
Ploidy of MKs from mouse bone marrow or from culture (day 3 or day 4) was measured as previously reported.8
Immunofluorescence
Results and discussion
Persistence of NMII-B expression during MK differentiation induces a decreased MK polyploidization
The mouse line, in which the NMII-A first coding exon has been disrupted by cDNA encoding GFP-tagged IIB, was used to check the specific role of NMII-B on polyploidization. The complete substitution of NMII-A by II-B (homozygous mice) was lethal at embryonic day 9.5 to 10.5.10 However, heterozygous A+/Ab* mice, which have persistent MYH10 expression associated with haploinsufficiency in MYH9 during MK differentiation, could provide an interesting model to study the specific role of NMII-B in MK polyploidization because mice with an MK-restricted myh9 deficiency have normal polyploidization.15 Therefore, if heterozygous (A+/Ab*) mice present with alterations in MK ploidy level, this will be attributable to the abnormal MYH10 expression, but not the MYH9 haploinsufficiency.
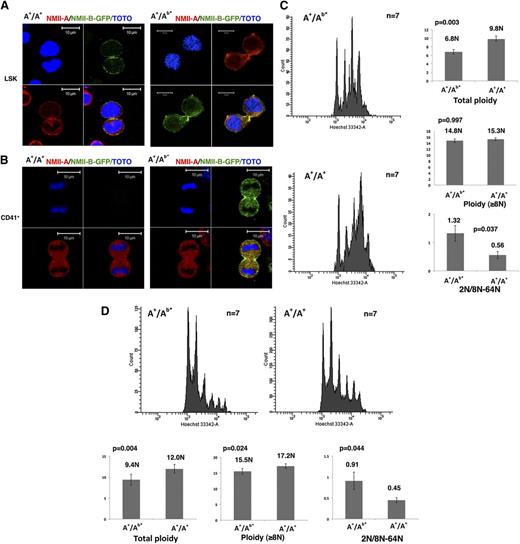
First, in heterozygous (A+/Ab*) or WT (wild type, A+/A+) LSK cells, both NMII-B and NMII-A were localized in the contractile ring (Figure 1A). Contrarily, in WT CD41+ MKs, both NMII-A and NMII-B were absent from the contractile ring (Figure 1B, left panel, and supplemental Figure 2A). However, for heterozygous MKs, only NMII-B was still accumulated in the contractile ring (Figure 1B, right panel). Therefore, in mice as in humans, only NMII-B, but not NMII-A, has the property to accumulate in the MK contractile ring. However, because MYH10 is silenced much faster during MK differentiation in mice compared with humans (supplemental Figure 2B),8 no myosin II accumulates in the contractile ring of WT murine MKs.
The persistent NMII-B expression during MK differentiation induces a decreased MK polyploidization. (A) NMII-B (green) as well as NMII-A (red) are present in the contractile ring of LSK cells obtained from A+/A+ WT mice (left) and A+/Ab* heterozygote mice (right). Bars indicate 10 µm. At least 30 dividing MKs were observed with 6 heterozygous and WT mice in 3 independent experiments. (B) No specific accumulation of NMII-A and NMII-B is observed in the contractile ring of CD41+ MKs from A+/A+ WT mice (left). On the contrary, CD41+ MKs derived from A+/Ab* heterozygous mice (right) exhibited a specific localization of NMII-B-GFP (green) in the contractile ring, but without accumulation of NMII-A (red). Bars indicate 10 µm. At least 30 dividing MKs were observed with 6 heterozygotes and 6 WT mice in 3 independent experiments. (C) Persistence of NMII-B expression during MK differentiation decreases the bone marrow MK ploidy level (in vivo) (left). Histograms show the total ploidy, the ploidy level of MKs ≥8N, and the ratio between 2N and 8N to 64N of bone marrow MKs from A+/Ab* heterozygous mice and control WT mice (A+/A+) (right). (D) Persistence of NMII-B expression during MK differentiation decreases the ploidy level of day-3 cultured MKs (in vitro) (top). Histograms show the total ploidy, the ploidy level of MKs ≥8N, and the ratio between 2N and 8N to 64N of cultured MKs derived from bone marrow of A+/Ab* heterozygous mice and control WT mice (A+/A+) (bottom).
The persistent NMII-B expression during MK differentiation induces a decreased MK polyploidization. (A) NMII-B (green) as well as NMII-A (red) are present in the contractile ring of LSK cells obtained from A+/A+ WT mice (left) and A+/Ab* heterozygote mice (right). Bars indicate 10 µm. At least 30 dividing MKs were observed with 6 heterozygous and WT mice in 3 independent experiments. (B) No specific accumulation of NMII-A and NMII-B is observed in the contractile ring of CD41+ MKs from A+/A+ WT mice (left). On the contrary, CD41+ MKs derived from A+/Ab* heterozygous mice (right) exhibited a specific localization of NMII-B-GFP (green) in the contractile ring, but without accumulation of NMII-A (red). Bars indicate 10 µm. At least 30 dividing MKs were observed with 6 heterozygotes and 6 WT mice in 3 independent experiments. (C) Persistence of NMII-B expression during MK differentiation decreases the bone marrow MK ploidy level (in vivo) (left). Histograms show the total ploidy, the ploidy level of MKs ≥8N, and the ratio between 2N and 8N to 64N of bone marrow MKs from A+/Ab* heterozygous mice and control WT mice (A+/A+) (right). (D) Persistence of NMII-B expression during MK differentiation decreases the ploidy level of day-3 cultured MKs (in vitro) (top). Histograms show the total ploidy, the ploidy level of MKs ≥8N, and the ratio between 2N and 8N to 64N of cultured MKs derived from bone marrow of A+/Ab* heterozygous mice and control WT mice (A+/A+) (bottom).
We thus wondered if NMII-B persistence in the A+/Ab* MKs could impair the switch from mitosis to endomitosis and inhibit MK polyploidization. In vivo analysis revealed a significantly lower MK ploidy level of heterozygous mice (A+/Ab*) (6.8N) than WT mice (A+/A+) (9.8N, n = 7, P = .003). Interestingly, the heterozygous mice have more 2N MKs compared with WT control. The ratio between 2N MKs and 8N to 64N MKs was much higher in A+/Ab* (1.32) vs WT (0.56, P = .037) (Figure 1C). This implies that NMII-B persistence favors mitosis (4N returns to 2N) in the switch between mitosis/endomitosis. However, the ploidy level measured in MKs ≥8N from A+/Ab* mice (14.8N) was not lower than in WT (15.3N). Similar results were found in MKs obtained by in vitro culture (Figure 1D). Altogether these results suggest that once MKs enter endomitosis, MYH10 persistence does not alter the subsequent polyploidization. These results are in agreement with those obtained by Gao et al, which revealed a different regulation for the first cycle of polyploidization (2N to 4N) from the subsequent cycles.7 MYH10 downregulation is thus only important for the first endomitosis cycle (ie, the switch from mitosis to endomitosis), as previously suggested by human MK culture.8
The C-terminal domain of MYH10 is responsible for the specific localization of myosin II in MK contractile ring
We then tried to understand why only NMII-B, but not NMII-A, is recruited to the contractile ring by studying another transgenic mouse line in which the N-terminal domain of GFP-tagged NMHCII-A is fused to the C-terminal II-B domain (Aab/Aab mice, chimeric hNMHCII-AB protein). The homozygous genotype is also lethal between embryonic day 11.5 and 12.5, and thus only heterozygous mice could be studied.11
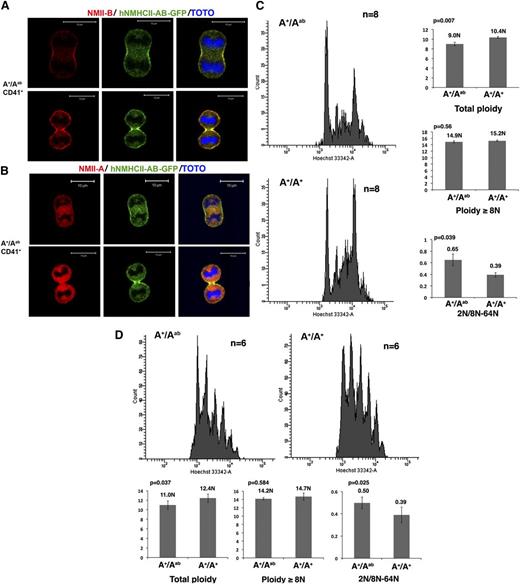
We first checked if the chimeric protein accumulates in the contractile ring. Contrary to WT MKs, which presented neither NMII-A nor NMII-B in the midzone (supplemental Figures 2 and 3), a specific localization of hNMHCII-AB in the contractile ring was revealed either by GFP expression or by an antibody recognizing NMII-B C terminus (Figure 2A-B). This provides strong evidence that localization of myosin II-B in the MK contractile ring depends on the C terminus of the heavy chain. Indeed, NMII-B and NMII-A share 79% sequence amino acid identity, and the majority difference is within the C-terminal tail. As demonstrated by in vitro studies, the small portion of the extreme C terminus, which clusters significantly the differences in primary structure of NMII-A and NMII-B, is particularly important for NMII isoform-specific distribution and assembly (solubility).12,13 Here, we provide the in vivo evidence supporting the idea that the C-terminal region of NMII regulates the isoform-distinct distribution.
The C-terminal domain determines the myosin II–specific localization in the contractile ring and its role in polyploidization. (A) CD41+ MKs from A+/Aab heterozygous mice exhibit a specific localization of chimeric hNMHCII-AB protein in the contractile ring as revealed by an antibody against the C terminus of NMII-B (red) or by the GFP expression (green). Bars indicate 10 µm. At least 30 dividing MKs were observed with 6 heterozygous and WT mice in 3 independent experiments. (B) An antibody against the C terminus of NMII-A revealed no specific localization of NMII-A tail (red) in the contractile ring of CD41+ MKs from A+/Aab heterozygous mice. Bars indicate 10 µm. At least 30 dividing MKs were observed with 6 heterozygous and WT mice in 3 independent experiments. (C) Expression of the chimeric hNMHCII-AB protein induces a decreased ploidy level of bone marrow MKs (left). Histograms show the total ploidy, the ≥8N ploidy level, and the ratio between 2N and 8N to 64N of bone marrow MKs from A+/Aab heterozygous mice and control WT mice (right). (D) Expression of the chimeric hNMHCII-AB protein induces a decreased ploidy level of day-4 cultured MKs from A+/Aab heterozygous mice compared with WT control mice (A+/A+) (top). Histograms show the total ploidy, the ≥8N ploidy level, and the ratio between 2N and 8N to 64N cultured MKs (bottom).
The C-terminal domain determines the myosin II–specific localization in the contractile ring and its role in polyploidization. (A) CD41+ MKs from A+/Aab heterozygous mice exhibit a specific localization of chimeric hNMHCII-AB protein in the contractile ring as revealed by an antibody against the C terminus of NMII-B (red) or by the GFP expression (green). Bars indicate 10 µm. At least 30 dividing MKs were observed with 6 heterozygous and WT mice in 3 independent experiments. (B) An antibody against the C terminus of NMII-A revealed no specific localization of NMII-A tail (red) in the contractile ring of CD41+ MKs from A+/Aab heterozygous mice. Bars indicate 10 µm. At least 30 dividing MKs were observed with 6 heterozygous and WT mice in 3 independent experiments. (C) Expression of the chimeric hNMHCII-AB protein induces a decreased ploidy level of bone marrow MKs (left). Histograms show the total ploidy, the ≥8N ploidy level, and the ratio between 2N and 8N to 64N of bone marrow MKs from A+/Aab heterozygous mice and control WT mice (right). (D) Expression of the chimeric hNMHCII-AB protein induces a decreased ploidy level of day-4 cultured MKs from A+/Aab heterozygous mice compared with WT control mice (A+/A+) (top). Histograms show the total ploidy, the ≥8N ploidy level, and the ratio between 2N and 8N to 64N cultured MKs (bottom).
Moreover, in vivo and in vitro MK ploidy analyses showed that hNMHCII-AB accumulation in the contractile ring decreased total MK ploidy level without modifying the ploidy level of MKs ≥8N. The 2N MK group in the heterozygous A+/Aab mice was also increased compared with WT control, which is confirmed by the ratio between 2N MKs and 8N to 64N MKs (Figure 2C, D). So, when the NMII-A N-terminal domain is properly localized in the MK contractile ring, it is capable of impairing the switch from mitosis to endomitosis, but in a less efficient manner than the NMII-B N-terminal domain, though both of them catalyze adenosine triphosphate hydrolysis. In fact, modifications in total ploidy level and the 2N MK/8N to 64N MK ratio observed between A+/Aab mice and their respective controls were less significant than those observed between A+/Ab* mice and their controls. This may be related to the different kinetic properties of NMII-A and NMII-B, which increase the fitness of NMII-B for cytokinesis.10,11,16-18
Together, the work revealed the important role of myosin II during MK mitosis/endomitosis transition and the critical role of NMII-B silencing for polyploidization. A greater understanding of the recruiting mechanisms of NMII-A and NMII-B in the MK contractile ring will help us to better comprehend MK polyploidization and its regulation, as well as the normal cytokinesis process. This work also underscores the importance of the C terminus in NMII function. Mutations in both the C terminus and N terminus of the NMII-A gene can be observed in the inherited human macrothrombocytopenia, MYH9-related diseases. A better understanding of the different NMII domain functions will be helpful to better understand the molecular mechanism of MYH9-related diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Françoise Wending, Robert S. Adelstein, and Mary Anne Conti reviewed the manuscript.
This work was supported by INSERM, by the Agence Nationale de la Recherche (ANR Jeune Chercheur) (Y.C.), and by grants from la Ligue Nationale Contre le Cancer (Equipe labellisée). I.B. was supported by ANR, and J.P. by the China Scholarship Council and la Société Française d'Hématologie. A.R. is supported by a grant from la Fondation de la Recherche Médicale (FRM).
Authorship
Contribution: I.B., J.P., C.L., S.B., A.R., and L.L. performed research; A.W. generated the 2 mouse lines; Y.C. designed and performed research; W.V. designed the research; and Y.C. wrote the paper with the help of W.V. and I.B.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yunhua Chang, INSERM UMR1009, Institut Gustave Roussy, Villejuif, France; e-mail: Yunhua.chang@gustaveroussy.fr or yunhua.chang-marchand@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal