Key Points
The synthetic retinoid ST1926 induces apoptosis of ATL cells and prolongs survival of ATL mice.
At the molecular level, ST1926 causes early DNA damage, upregulates p53, and downregulates Tax expression.
Abstract
Adult T-cell leukemia/lymphoma (ATL) is an aggressive neoplasm caused by human T-cell leukemia virus type 1 (HTLV-1). The HTLV-1 oncoprotein Tax plays an important role in ATL pathogenesis. ATL carries a poor prognosis due to chemotherapy resistance, stressing the need for alternative therapies. Here, we investigate the preclinical efficacy of the synthetic retinoid ST1926 in ATL and peripheral T-cell lymphomas. Clinically achievable concentrations of ST1926 induced a dramatic inhibition of cell proliferation in malignant T-cell lines and primary ATL cells with minimal effect on resting or activated normal lymphocytes. ST1926 induced apoptosis, DNA damage, and upregulation of p53 proteins in malignant T cells, whereas it caused an early downregulation of Tax proteins in HTLV-1–positive cells. In murine ATL, oral treatment with ST1926 prolonged survival and reduced leukemia cell infiltration, white blood cell counts, and spleen mass. In spleens of ST1926-treated animals, p53 and p21 proteins were upregulated, poly (ADP-ribose) polymerase was cleaved, and Tax transcripts were reduced. These results highlight the promising use of ST1926 as a targeted therapy for ATL.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is an aggressive lymphoid proliferation1,2 associated with the oncoretrovirus human T-cell lymphotropic virus type 1 (HTLV-1).2,3 The viral transactivator oncoprotein Tax plays a key role in T-cell transformation and in deregulation of many cellular pathways. Tax activates the viral HTLV-1 promoter and various cellular genes, deregulates apoptosis, cell cycle, and DNA repair, and is implicated in ATL leukemogenesis.2,4
ATL develops after a long latency period and carries a very poor prognosis due to chemotherapy resistance and profound immunosuppression.5-8 The combination of zidovudine and interferon (IFN)α induces a high response rate and prolongs survival of ATL patients.9-12 Moreover, the triple combination of arsenic trioxide (AsO3), zidovudine, and IFN triggers complete and durable clinical remission in ATL patients.13 Unfortunately, most of the patients relapse, stressing the need for alternative or complementary therapies.
Retinoids are powerful regulators of hematopoietic cell growth and differentiation. Vitamin A derivatives, such as all-trans retinoic acid (ATRA), are used in the treatment of certain leukemias, mainly acute promyelocytic leukemia (APL).14,15 The use of retinoids was also investigated in ATL. Significant growth inhibition by ATRA was observed in some HTLV-1–positive cell lines,16 whereas resistance was noted in others.17 Primary ATL cells from several patients displayed variable responses to ATRA, and ATL cells from some ATRA-sensitive patients later developed resistance.18 Finally, ATRA was clinically evaluated in 20 ATL patients and showed no complete remission but a partial response in 40% of the patients.19
Even though natural retinoids have been used in APL treatment, their broad clinical use has been hampered by side effects and resistance. In addition, retinoid receptor signaling pathway is deregulated in several cancers, making tumor cells resistant to natural retinoids.20,21 Thus, synthetic retinoids were developed with increased specificity and decreased toxicity. One promising compound is the synthetic adamantly retinoid, ST1926 (2E)-3-[3′-(1-adamantyl)-4′-hydroxy[1,1′-biphenyl]-4-yl]-2-propenoic acid, an analog of CD437 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid.22 ST1926 showed potential efficacy in solid tumors and hematological malignancies with minimal side effects.23,24 Furthermore, oral ST1926 is pharmacokinetically stable, bioavailable,23 and pharmacologically attainable in the plasma of patients at micromolar concentrations.24 Therefore, this compound entered phase 1 clinical trials.25,26
We investigated the efficacy of ST1926 in ATL models in vitro and in vivo. We show that pharmacologically achievable concentrations of ST1926 are potent inducers of cell death in ATRA-resistant HTLV-1–positive and –negative malignant T-cell lines and of primary ATL cells, with no effect on resting or activated normal lymphocytes. In murine ATL, ST1926 reduced tumor burden and prolonged survival. At the molecular level, ST1926 decreased the levels of Tax expression in vitro and in vivo. Hence, ST1926 is a promising synthetic retinoid in ATL-targeted therapy.
Materials and methods
Cell lines, drugs, and culture conditions
The HTLV-1–transformed CD4+ T-cell lines (HuT-102, MT-2, and C8166) and the HTLV-1–negative CD4+ leukemic cell lines (CEM, Jurkat, and MOLT-4) were grown at 2 × 105 cells/mL as previously described.27 Peripheral blood mononuclear cells (PBMCs) were collected from 3 healthy HTLV-1–negative donors and 3 acute ATL patients and centrifuged over Ficoll-Hypaque (Lymphoprep, Nyegaard, Norway). Activated PBMCs were grown as previously described.17 Primary ATL cells were thawed with a viability of 70% to 80% and cultured at 2 × 105 viable cells/mL.
The synthetic retinoid CD437 was kindly provided by Sigma-Tau (Rome, Italy) or purchased from Sigma Chemical Co. (St. Louis, MO). ST1926, a CD437 analog, was kindly provided by Sigma-Tau and Biogem (Ariano Irpino, Italy). The synthetic retinoid N-(4-hydroxyphenyl)retinamide (HPR) was purchased from Sigma Chemical Co. Retinoids were reconstituted in dimethylsulfoxide (DMSO) at a concentration of 1 × 10−2 M and stored at −80οC. The final concentrations of DMSO never exceeded 0.1%, which showed no effect on the proliferation of all tested cells. The caspase inhibitor z-VAD (Bachem Bioscience) was added 2 hours prior to ST1926 at a final concentration of 100 µM.
Growth assays and cell cycle analysis
Cell growth was assessed by the use of the CellTiter 96 nonradioactive cell proliferation assay kit based on the MTT assay (Promega Corp., Madison, WI), according to the manufacturer’s instructions and by the trypan blue dye exclusion protocols. Cell cycle analysis was performed using propidium iodide (50 μg/mL; Sigma-Aldrich) staining and a FACScan flow cytometer (Becton Dickinson), as previously described.17
Terminal deoxy-transferase–mediated deoxyuridine triphosphate nick-end labeling assay
The terminal deoxy-transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) assay was used according to the manufacturer's suggestions (Boehringer, Mannheim, Germany). Fluorescein-conjugated dUTP incorporated in nucleotide polymers was detected and quantified using flow cytometry. Approximately 10 000 cells per sample were acquired and analyzed using Cell-Quest software (Becton-Dickinson).
Immunoblot analysis
Total protein extracts from treated and untreated cells in culture or from frozen cells from spleens of mice were prepared as previously described.17 Protein quality and equal loading were assessed by Ponceau S staining and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) immunoblotting. Bands were quantified using Image J software (National Institutes of Health, Bethesda, MD). Antibodies were purchased for caspase-3 (sc-7148), p21 (sc-397), and p53 (murine p53 sc-6243, human p53 sc-126) (Santa Cruz Biotechnology, Santa Cruz, CA); poly (ADP-ribose) polymerase (9542), phosphorylated-p53 (9284), and γ-H2AX (2577) (Cell Signaling, Danvers, MA); and GAPDH (MAB5476) (Abnova, Heidelberg, Germany). Mouse monoclonal anti-Tax (168-A51) was obtained from the National Institutes of Health AIDS Research and Reagent Program.
Mice
We used the ATL mouse model obtained by Hasegawa et al.28 To test for the effect of ST1926, we established a rapid and reproducible model of disease by direct intraperitoneal transfer of 106 spleen cells from Tax transgenic mice into SCID mice (Charles River). Mice protocols were approved by the Institutional Animal Care and Utilization Committee of the American University of Beirut. Animals were housed in specific pathogen-free housing and were euthanized by cervical dislocation following deep anesthesia with isoflurane.
In vivo treatment
ST1926 was prepared as stock solution 10−2 M in DMSO and stored in amber tubes at −80°C. Stock ST1926 solution was diluted in 1:1 cremophor/ethanol solution (10 µL stock ST1926 containing 0.6 mg in 40 µL cremophor/ethanol). Each animal received by gavage 50 µL of ST1926 solutions, equivalent to 30 mg/kg body weight (average mouse weight, 20 g) as this dosage was reported to cause survival advantage in AML mice with no signs of toxicity.24 For survival experiments, 2 ST1926 treatment protocols (30 mg/kg, by gavage) were tested: the first consisted of ST1926 twice a day for 2 consecutive weeks (28 doses), and the second consisted of ST1926 once every other day for 3 consecutive weeks (9 doses). Both treatment protocols showed no toxicity in normal SCID mice except for a slight decrease in their weight (<10%). In addition, we checked several organs (lung, liver, spleen, and kidney) and their histological architecture was normal in hematoxylin and eosin-stained sections. Both protocols were initiated at day 6 after intraperitoneal injection of malignant cells from Tax-transgenic mice following our optimized protocols in ATL mice.29 To test for the effect of treatment on organ infiltration and ATL molecular markers, the second protocol was used, and treated animals were euthanized at the same time as untreated controls. Tissues from both ST1926-treated and untreated control mice were fixed as previously described.29
Quantitative polymerase chain reaction
Spleens from ST1926-treated and untreated control mice were harvested at different time points. LightCycler (Roche Diagnostics) and LightCycler v4.05 software were used to quantitate the absolute Tax DNA content and the relative Tax mRNA expression by real-time polymerase chain reaction (PCR; LightCycler Fast Start DNA Master kit) and by reverse transcription PCR (LightCycler RNA Master kit), respectively. DNA was extracted using the QIAamp DNA Mini Kit (Qiagen), whereas RNA was extracted by the TRIZOL reagent (Life Technologies) as previously described.29
The forward primers for SK-43 (5′-CGGATACCCAGTCTACGTGT-3′) and SK-44 (5′-GAGCCGATAACGCGTCCATCG-3′), and the reverse-labeled primers for SK4I-FL (5′-CCCTACTGGCCACCTGTCCAGAGC- FL-3′) and SK4I-LC (5′-LC Red640-TCAGA TCACCTGGGACCCCATCPH- 3′) were designed in collaboration with Tib-MolBiol (Germany) to span the Tax region of the HTLV-1 genome. The same primers were used for real-time PCR for both RNA and DNA experiments.
Statistical analysis
Survival curves were calculated according to the methods of Kaplan and Meier. Overall survival is defined as time from injection of ATL cells to death from any cause. Mice that were still alive were censored at the time they were last known to be alive. SPSS (version 16.0) and Microsoft Office Excel 2010 were used for statistical analyses. In scatter plots, analyzed samples fit normal distributions, and means were compared using an independent sample Student t test. In survival analyses, median values were reported and compared using log rank, Breslow, and Tarone-Ware tests, with similar results. Statistical significance was reported when P ≤ .05.
Results
ST1926 induces growth arrest in malignant T cells
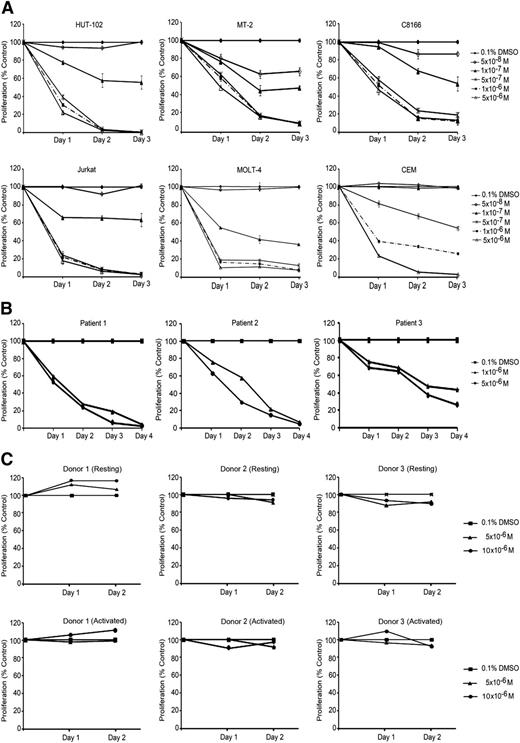
We used 3 HTLV-1–transformed cell lines (HuT-102, MT-2, and C8166) and 3 HTLV-1–negative cell lines (CEM, Jurkat, and MOLT-4) to test for the effects of ST1926 on cell growth and viability. We previously reported that most of these cell lines are resistant to ATRA.17,27 We used ST1926 concentrations ranging from 0 05 to 5 µM as these are pharmacologically achievable.26 ST1926 treatment resulted in a time-dependent growth inhibition of all tested cell lines. In general, both HTLV-1–negative and –positive cell lines were equally sensitive to ST1926 (Figure 1A). A threshold level at 0.5 µM concentration was noted, with similar growth suppressive effects as 10-fold higher concentrations, except for CEM cells (Figure 1A).
ST1926 induces growth arrest in malignant T cells. (A) Effects of ST1926 treatment on the growth of HTLV-1–positive (HuT-102, MT-2, and C8166) and HTLV-1–negative (Jurkat, CEM, and MOLT-4) malignant cell lines: cell growth was assayed in quadruplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. Viability results are expressed as percentage of control (0.1% DMSO) and represent the mean of ≥3 independent experiments ± standard error (SE). (B) Primary ATL cells are sensitive to ST1926: primary ATL from 3 patients (patients 1 and 2 newly diagnosed acute ATL and patient 3 relapsed acute ATL) were treated with the indicated concentrations of ST1926, and cell growth was assayed in quadruplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. The results are expressed as percentage of control (0.1% DMSO) ± standard deviation (SD) and are representative of 2 independent experiments. (C) Resting and activated T-lymphocytes are resistant to suprapharmacological concentrations of ST1926. PBMCs were collected from 3 healthy donors. Activated PBMCs were supplemented with 2% PHA. Cells were seeded in 24-well plates and treated with 0.1% DMSO or 5 to 10 μM ST1926 up to 48 hours. Cell growth was assayed in triplicate wells using the Cell Titer 96 nonradioactive cell proliferation kit. Results are expressed as percentage of control (0.1% DMSO) ± SD.
ST1926 induces growth arrest in malignant T cells. (A) Effects of ST1926 treatment on the growth of HTLV-1–positive (HuT-102, MT-2, and C8166) and HTLV-1–negative (Jurkat, CEM, and MOLT-4) malignant cell lines: cell growth was assayed in quadruplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. Viability results are expressed as percentage of control (0.1% DMSO) and represent the mean of ≥3 independent experiments ± standard error (SE). (B) Primary ATL cells are sensitive to ST1926: primary ATL from 3 patients (patients 1 and 2 newly diagnosed acute ATL and patient 3 relapsed acute ATL) were treated with the indicated concentrations of ST1926, and cell growth was assayed in quadruplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. The results are expressed as percentage of control (0.1% DMSO) ± standard deviation (SD) and are representative of 2 independent experiments. (C) Resting and activated T-lymphocytes are resistant to suprapharmacological concentrations of ST1926. PBMCs were collected from 3 healthy donors. Activated PBMCs were supplemented with 2% PHA. Cells were seeded in 24-well plates and treated with 0.1% DMSO or 5 to 10 μM ST1926 up to 48 hours. Cell growth was assayed in triplicate wells using the Cell Titer 96 nonradioactive cell proliferation kit. Results are expressed as percentage of control (0.1% DMSO) ± SD.
We also determined the effect of ST1926 on PBMCs isolated from 3 patients: 2 with newly diagnosed acute ATL and 1 with chronic ATL in relapse30 (unpublished results). Interestingly, 1 µM ST1926 caused a growth inhibition that was almost complete in primary ATL cells from the newly diagnosed acute ATL patients and >60% in the relapsed one (Figure 1B). In contrast, resting and phytohemagglutinin-stimulated normal T cells from 3 healthy donors were resistant to ST1926 up to 10 μM (Figure 1C).
Because ST1926 is an analog of CD437, we compared the growth suppressive effects of these retinoids. CD437 treatment resulted in a dose- and time-dependent growth inhibition of all tested cell lines and primary ATL cells (supplemental Figure 1A-B), whereas no effect was observed on resting or PHA-activated PBMCs (supplemental Figure 1C). Furthermore, HTLV-1–negative cell lines were more sensitive to CD437 treatment. Interestingly, ST1926 treatment was generally found to be 2- to 10-fold more potent than CD437 in decreasing the growth of malignant T-cell lines or primary ATL cells (Figure 1; supplemental Figure 1).
ST1926 causes G1 cell cycle arrest and massive apoptosis in malignant T cells
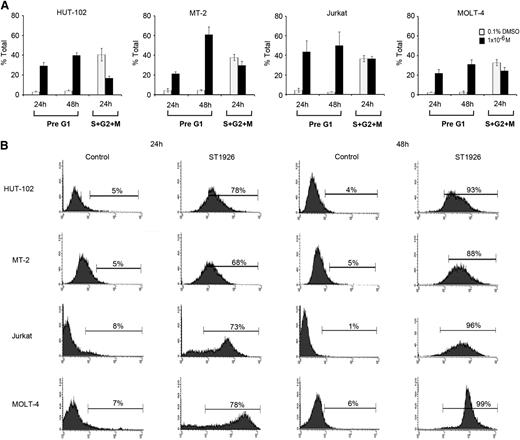
To investigate the mechanisms involved in ST1926-induced growth inhibition and cell death, cell cycle analysis was performed after treating the HTLV-1–positive cell lines (HuT-102 and MT-2) and the HTLV-1–negative cell lines (Jurkat and Molt-4) with 1 μM ST1926 up to 48 hours. This later concentration is pharmacologically achievable and causes ≥90% growth inhibition at 48 hours in these cells.
No major variation in cell cycle distribution was observed between untreated HuT-102, MT-2, Jurkat, and Molt-4 cells (Figure 2A; supplemental Figure 2). Twenty-four hours after treatment, ST1926 induced a major increase in the presumably apoptotic pre-G1 region in tested cells (Figure 2A) and a modest G1 cell cycle arrest, because cycling cells diminished by 59%, 21%, and 27% in HuT-102, MT-2, and Molt-4 cells, respectively (Figure 2A).
ST1926 induces cell cycle arrest and massive apoptosis in malignant T cells. (A) Effects of ST1926 on the cell cycle distribution of HuT-102, MT-2, Jurkat, and MOLT-4 cells. Cells were treated with 1 μM ST1926 up to 48 hours and stained with propidium iodide (50 μg/mL), and cell cycle analysis was performed using a FACScan flow cytometer. The pre-G1 percentage represents apoptotic cells. Cycling cells, the sum of (S + G2/M) phases, are a percentage of nonapoptotic cells. Percentage cells in G1 phase are calculated as 100 − (S + G2/M). The results represent the average of 3 independent experiments ± SE. (B) TUNEL analysis of HuT-102, MT-2, Jurkat, and MOLT-4 cells treated for 48 hours with 1 μM ST1926 concentrations. The results are representative of 2 independent experiments.
ST1926 induces cell cycle arrest and massive apoptosis in malignant T cells. (A) Effects of ST1926 on the cell cycle distribution of HuT-102, MT-2, Jurkat, and MOLT-4 cells. Cells were treated with 1 μM ST1926 up to 48 hours and stained with propidium iodide (50 μg/mL), and cell cycle analysis was performed using a FACScan flow cytometer. The pre-G1 percentage represents apoptotic cells. Cycling cells, the sum of (S + G2/M) phases, are a percentage of nonapoptotic cells. Percentage cells in G1 phase are calculated as 100 − (S + G2/M). The results represent the average of 3 independent experiments ± SE. (B) TUNEL analysis of HuT-102, MT-2, Jurkat, and MOLT-4 cells treated for 48 hours with 1 μM ST1926 concentrations. The results are representative of 2 independent experiments.
To confirm apoptosis, we used the TUNEL assay. Treatment of malignant T-cell lines with 1 μM ST1926 caused a substantial increase in TUNEL positivity reaching ≥68% of all tested cells by 24 hours (Figure 2B). Conversely, CD437 caused a more pronounced G1 cell cycle arrest but a lesser induction of apoptosis than ST1926 (data not shown).
ST1926-induced apoptosis in malignant T cells is partially caspase dependent
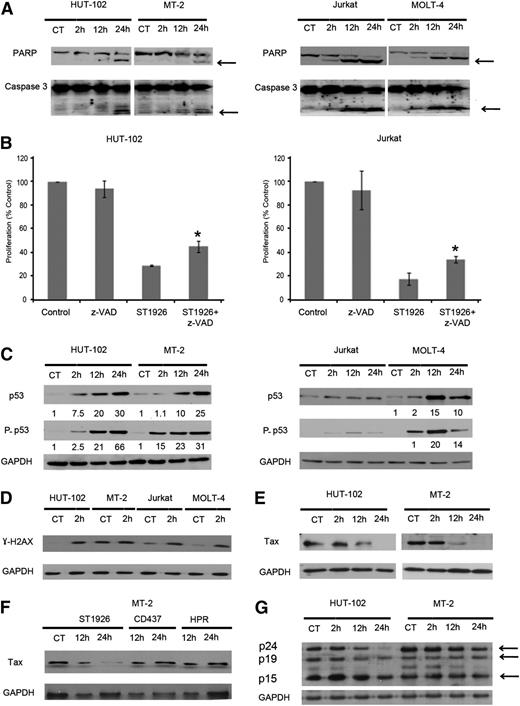
We then investigated whether caspases are involved in ST1926-induced cell death. Therefore, we treated the HTLV-1–positive (HuT-102 and MT-2) and HTLV-1–negative (Jurkat and MOLT-4) cells with 1 µM ST1926 up to 48 hours. ST1926-induced apoptosis was associated with caspase activation, as shown by PARP cleavage (113 kDa) into its death-associated fragments (89 and 24 kDa) in all tested cells (Figure 3A). PARP cleavage was clearly observed at 12 hours in HuT-102– and MT-2–treated cells and as early as 2 hours in Jurkat- and MOLT-4–treated cells. Caspase 3 was also cleaved in all tested cells (Figure 3A). To determine whether ST1926-induced apoptosis is caspase dependent, we pretreated HuT-102 and Jurkat cells with z-VAD, a pan-caspase inhibitor for 2 hours, prior to 24-hour treatment with 1 µM ST1926. ST1926-induced growth inhibition in both cell lines was partially and significantly reversed after z-VAD pretreatment (Figure 3B). In HuT-102 and Jurkat cells, 1 µM ST1926 decreased growth to 39% and 18% after 24 hours of treatment, respectively, and in the presence of z-VAD, cell growth increased back to 55% and 66%, respectively (P < .05). This indicates that ST1926-induced cell death is partially caspase dependent. Similarly CD437-induced growth inhibition of malignant T cells was found to be partially caspase dependent (data not shown).
ST1926 induces partially caspase-dependent apoptosis and results in elevated levels of p53 and γ-H2AX proteins and in reduced Tax protein levels. (A) ST1926 causes PARP and caspase 3 cleavage in HuT-102, MT-2, Jurkat, and MOLT-4 cells. Total sodium dodecyl sulfate protein lysates (50 µg/lane) were prepared after treating malignant T cells with 1 μM ST1926 for up to 48 hours. Lysates were immunoblotted against PARP and caspase-3 antibodies. Arrows indicate cleaved PARP and caspase 3. (B) Effects of the general caspase inhibitor z-VAD on ST1926-induced growth inhibition of HuT-102, MT-2, Jurkat, and MOLT-4 cells. Cells were pretreated for 2 hours with 100 μM z-VAD, followed by 24-hour treatment with 1 μM ST1926. Cell viability was determined in quadruplicate wells by the trypan blue dye exclusion and expressed as percentage of control. Results are an average of 2 independent experiments ± SE. *Statistically significant (P < .05) differences (independent t test). (C) ST1926 upregulates p53 and its phosphorylated form (P-p53) in malignant T cells. HuT-102, MT-2, Jurkat, and MOLT-4 cells were treated with 1 μM ST1926 for up to 48 hours and immunoblotted against p53 and P-p53 antibodies. Similar trends in protein levels were observed in 2 independent experiments. Bands were quantified, and results are expressed relative to control cells or 2-hour time point. (D) ST1926 elicits an early DNA damage response. Cells were treated with 1 μM ST1926 and immunoblotted against γ-H2AX antibodies at 2 hours after treatment. Similar trends in protein levels were observed in 2 independent experiments. (E) ST1926 reduces Tax protein levels in treated HuT-102 and MT-2 cells. Cells were treated with 1 μM ST1926 for up to 24 hours and immunoblotted against Tax antibodies. Similar trends in protein levels were observed in 3 independent experiments. All blots were reprobed with GAPDH antibody to ensure equal protein loading. (F) ST1926, but not CD437 or HPR, decreases Tax protein levels in treated MT-2 cells. Cells were treated with 1 μM ST1926 for up to 24 hours and immunoblotted against Tax antibodies. Similar trends in protein levels were observed in 2 independent experiments. All blots were reprobed with GAPDH antibody to ensure equal protein loading. (G) ST1926 reduces gag (p24, p19, and p15) protein levels in treated HuT-102 and MT-2 cells. Cells were treated with 1 μM ST1926 at the indicated time points and immunoblotted against sera derived from ATL patients.
ST1926 induces partially caspase-dependent apoptosis and results in elevated levels of p53 and γ-H2AX proteins and in reduced Tax protein levels. (A) ST1926 causes PARP and caspase 3 cleavage in HuT-102, MT-2, Jurkat, and MOLT-4 cells. Total sodium dodecyl sulfate protein lysates (50 µg/lane) were prepared after treating malignant T cells with 1 μM ST1926 for up to 48 hours. Lysates were immunoblotted against PARP and caspase-3 antibodies. Arrows indicate cleaved PARP and caspase 3. (B) Effects of the general caspase inhibitor z-VAD on ST1926-induced growth inhibition of HuT-102, MT-2, Jurkat, and MOLT-4 cells. Cells were pretreated for 2 hours with 100 μM z-VAD, followed by 24-hour treatment with 1 μM ST1926. Cell viability was determined in quadruplicate wells by the trypan blue dye exclusion and expressed as percentage of control. Results are an average of 2 independent experiments ± SE. *Statistically significant (P < .05) differences (independent t test). (C) ST1926 upregulates p53 and its phosphorylated form (P-p53) in malignant T cells. HuT-102, MT-2, Jurkat, and MOLT-4 cells were treated with 1 μM ST1926 for up to 48 hours and immunoblotted against p53 and P-p53 antibodies. Similar trends in protein levels were observed in 2 independent experiments. Bands were quantified, and results are expressed relative to control cells or 2-hour time point. (D) ST1926 elicits an early DNA damage response. Cells were treated with 1 μM ST1926 and immunoblotted against γ-H2AX antibodies at 2 hours after treatment. Similar trends in protein levels were observed in 2 independent experiments. (E) ST1926 reduces Tax protein levels in treated HuT-102 and MT-2 cells. Cells were treated with 1 μM ST1926 for up to 24 hours and immunoblotted against Tax antibodies. Similar trends in protein levels were observed in 3 independent experiments. All blots were reprobed with GAPDH antibody to ensure equal protein loading. (F) ST1926, but not CD437 or HPR, decreases Tax protein levels in treated MT-2 cells. Cells were treated with 1 μM ST1926 for up to 24 hours and immunoblotted against Tax antibodies. Similar trends in protein levels were observed in 2 independent experiments. All blots were reprobed with GAPDH antibody to ensure equal protein loading. (G) ST1926 reduces gag (p24, p19, and p15) protein levels in treated HuT-102 and MT-2 cells. Cells were treated with 1 μM ST1926 at the indicated time points and immunoblotted against sera derived from ATL patients.
ST1926 treatment upregulates p53, causes a DNA damage response, and downregulates Tax
To decipher ST1926-mediated growth inhibition and cell death, p53 protein levels were monitored at 2, 12, and 24 hours following 1 µM treatment of malignant T-cell lines. ST1926 induced a substantial upregulation of total p53 proteins and of the p53 phosphorylated form at serine 15 as early as 2 hours in HuT-102, MT-2, and Molt-4 cells (Figure 3C). Similar increases were observed for total p53 levels in CD437-treated cells (data not shown). Conversely, p53 protein levels and phosphorylated form did not change in Jurkat cells, which possess a mutant p5331 (Figure 3C). To test whether ST1926 and CD437 are mediating their growth inhibitory effects through p53, we determined their antiproliferative effects on a p53-null cell line, MOLT-4-E6, and compared it to the MOLT-4 cells with wild-type p53.32 ST1926 and CD437 treatments resulted in similar dose- and time-dependent growth inhibition on both cell lines that have different p53 status (data not shown). To assess whether ST1926 reactivates p53 in HTLV-1–positive cells, we tested for several p53 target genes, namely p21, Bax, NOXA, and MDM2, in HuT-102 and MT-2 cells and did not observe any upregulation of protein levels on ST1926 treatment in these cell lines (data not shown), perhaps arguing against p53 reactivation.
These experiments suggest that the growth suppressive effects of these adamantyl retinoids in malignant T cells are p53 independent. Previous studies showed that ST1926 is a strong inducer of genotoxic stress and DNA damage.26,33,34 We observed a substantial increase in the DNA damage marker, H2AX phosphorylation (γ-H2AX), as early as 2 hours in tested cells (Figure 3D). Some tumor cells show elevated baseline levels of DNA damage35 as noted in MT-2 cells and to a lower extent in Jurkat and Molt-4 cells (Figure 3D), which may be due to several factors such as chromatin instability36 and damaged telomeres.37 Tax has been shown to increase genetic instability by inducing DNA double strand breaks as evidenced by increased γ-H2AX levels38 ; however, other factors may be also implicated as shown by the variability in the expression of endogenous γ-H2AX levels in HuT-102 and MT-2 cells.
We did not detect any changes in the protein levels of the apoptotic regulators Bcl-2, Bax, and Bak in ST1926- or CD437-treated cells (data not shown). Finally, in HuT-102 and MT-2 cells, Tax protein levels were reduced after 12 hours of ST1926 treatment and disappeared completely by 24 hours in both cell lines (Figure 3E). We treated MT-2 cells with ST1926, CD437, and HPR. We show that only ST1926 reduced Tax protein levels at pharmacologically achievable 1 µM concentrations (Figure 3F). To address whether ST1926 is inducing Tax-specific degradation or acts on other viral proteins, we tested sera from ATL patients on protein extracts from HuT-102 and MT-2 cell lines treated with ST1926 for 2, 24, and 48 hours. Interestingly, although the level of the 3 gag proteins (p24, p19, and p15) were reduced on treatment (Figure 3G), this decrease followed that of Tax, particularly in MT-2 cells (Figure 3F). These results suggest that Tax is the primary target, and a decrease in Tax levels will result in reduced viral expression. Finally, no significant cell death was observed due to ST1926 treatment up to 12 hours, whereas Tax protein levels were dramatically reduced (data not shown).
Altogether, these results show that ST1926 is a potent inducer of apoptosis and DNA damage in malignant T cells and reduces Tax oncoprotein levels in HTLV-1–positive cells that precedes cell death.
ST1926 prolongs survival and reduces leukemic cells infiltration in ATL mice
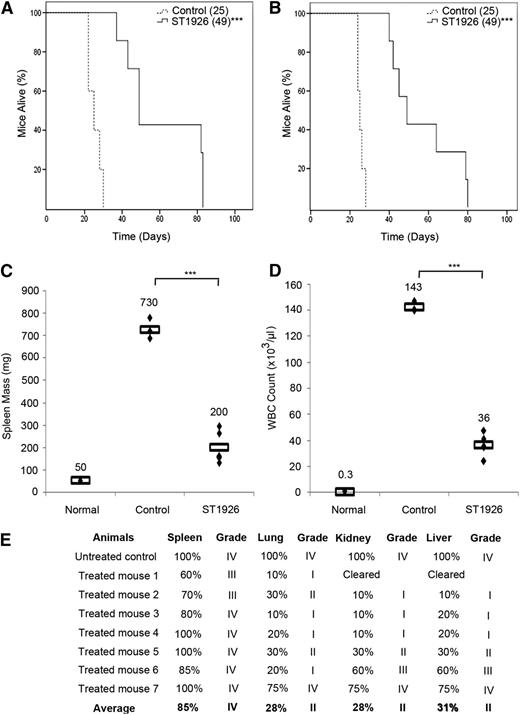
To assess the in vivo efficacy of ST1926, we reproduced the well-characterized ATL transplantation model where 106 ATL spleen cells from Tax transgenic mice28 are inoculated into SCID mice. Recipients, like the original transgenics, developed a lethal massive hyperleucocytosis, splenomegaly, hypercalcemia, and multiple organ invasion, especially in the spleen, liver, kidney, and lungs, and died within 24 to 28 days. To investigate any survival benefit of ST1926, we first treated leukemic mice with oral ST1926 twice per day with 30 mg/kg for 2 consecutive weeks. In comparison with untreated controls (median survival of 25 days), a significant survival advantage was seen in ST1926-treated animals, with a median survival of 49 days (P < .001; Figure 4A). Strikingly, ST1926 oral treatment of leukemic mice (30 mg/kg) for 9 or 28 doses resulted in the same survival advantage (median 49 days and P < .001; Figure 4A-B).
ST1926 treatment prolongs survival in an ATL mouse model. ATL mice treated orally with ST1926 at the dose of 30 mg/kg (A) twice daily for 2 consecutive weeks or (B) once every other day for 3 weeks. Kaplan-Meier plots of overall survival are displayed. Reported between parentheses are median survival times. ***P < .001. (C-E) Effect of 3 weeks of ST1926 treatment at 30 mg/kg every other day on (C) spleen mass, (D) circulating WBC count, and (E) organ infiltration by histopathology. Rectangles in C and D represent mean values. ***P < .001. Scores of organ infiltration after histopathology analysis are shown in E: score I, 1% to 25% leukemic cell infiltrate; score II, 26% to 50%; score III, 51% to 75%; score IV, 76% to 100%.
ST1926 treatment prolongs survival in an ATL mouse model. ATL mice treated orally with ST1926 at the dose of 30 mg/kg (A) twice daily for 2 consecutive weeks or (B) once every other day for 3 weeks. Kaplan-Meier plots of overall survival are displayed. Reported between parentheses are median survival times. ***P < .001. (C-E) Effect of 3 weeks of ST1926 treatment at 30 mg/kg every other day on (C) spleen mass, (D) circulating WBC count, and (E) organ infiltration by histopathology. Rectangles in C and D represent mean values. ***P < .001. Scores of organ infiltration after histopathology analysis are shown in E: score I, 1% to 25% leukemic cell infiltrate; score II, 26% to 50%; score III, 51% to 75%; score IV, 76% to 100%.
This survival advantage was accompanied by a sharp reduction of spleen mass in ST1926-treated animals after 3 weeks of treatment, with a mean mass decreasing from 730 to 200 mg (Figure 4C). In addition, the average number of circulating white blood cells (WBCs) significantly decreased from 143 × 103/μL to 36 × 103/μL (Figure 4C), and no obvious side effect was observed. Pathology examination demonstrated a gradual decrease in the leukemic cell infiltrates, especially within the liver, lung, and kidney in ST1926-treated animals (Figure 4D). Among 7 treated animals, 5 were good responders, where the kidney and liver were completely cleared in 1 animal and the grade of infiltration decreased from grade IV in untreated controls to grade II or lower in livers, lungs, and kidneys of this group of responders. In contrast, 2 mice responded less to ST1926 treatment (Figure 4E). In summary, ST1926 oral treatment significantly prolonged survival of ATL mice, decreased their spleen mass and circulating WBCs, and reduced leukemic cell infiltrations to several organs.
ST1926 decreases Tax mRNA and DNA and induces apoptosis in vivo
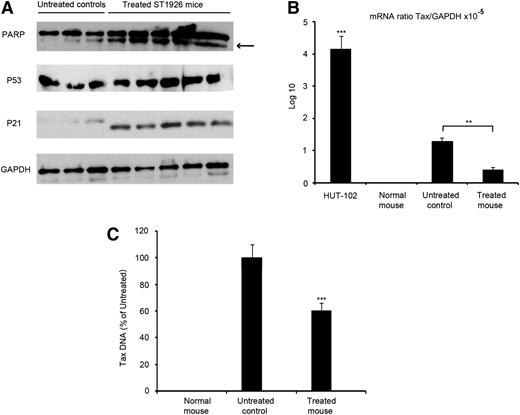
To dissect the molecular basis for the conferred survival advantage in ST1926-treated leukemic mice, we examined apoptosis markers in vivo. We harvested spleen leukemic cells from both untreated and treated ST1926 ATL mice. Consistent with the in vitro results in HTLV-1–transformed cell lines, ST1926 induced PARP cleavage in vivo, indicative of caspase activation and apoptosis induction (Figure 5A). Furthermore, p53 and p21 protein levels were up-regulated in leukemic cells derived from the spleen of treated animals compared with the untreated controls (Figure 5A). Tax was shown to induce apoptosis and elevated p53 protein levels in several lines of CD3-Tax-transgenic animals39 as also noted in the leukemic cells derived from the spleen of untreated animals (Figure 5A). Because the model we used is a SCID ATL model, we cannot check for the effect of ST1926 on lymphocytes of control-treated animals due to the lack of lymphocytes in the control SCID animals. We therefore treated normal balb-c mice with 30 mg/kg ST1926 3 times per week for 3 weeks, but we could not detect any upregulated levels of total p53 proteins in spleen lymphocytes from untreated or treated animals (data not shown).
ST1926 induces apoptosis and decreases Tax expression in vivo. (A) Western blot analysis for PARP, p53, p21, and GAPDH on spleen-derived leukemic cells from 3 untreated and 5 ST1926-treated mice at 30 mg/kg every other day for 3 weeks. (B) Average of Tax mRNA expression in spleen-derived leukemic cells from a normal mouse, 3 untreated controls, and 5 ST1926-treated mice. **P < .01, ***P < .001. (C) Quantitative PCR analysis of Tax DNA in spleen-derived leukemic cells from 3 untreated controls and 5 ST1926-treated mice. ***P < .001. Results are represented as percentage of untreated controls ± SD.
ST1926 induces apoptosis and decreases Tax expression in vivo. (A) Western blot analysis for PARP, p53, p21, and GAPDH on spleen-derived leukemic cells from 3 untreated and 5 ST1926-treated mice at 30 mg/kg every other day for 3 weeks. (B) Average of Tax mRNA expression in spleen-derived leukemic cells from a normal mouse, 3 untreated controls, and 5 ST1926-treated mice. **P < .01, ***P < .001. (C) Quantitative PCR analysis of Tax DNA in spleen-derived leukemic cells from 3 untreated controls and 5 ST1926-treated mice. ***P < .001. Results are represented as percentage of untreated controls ± SD.
Because Tax protein was undetectable in ATL mice,28 as in primary human ATLs,2 we measured Tax transcripts in harvested leukemic cells from both untreated and ST1926-treated ATL mice. Tax mRNA levels were almost 1000-fold higher in the HuT-102 cells than in the spleen cells of untreated ATL (Figure 5B).29 In coherence with the spleen mass decrease, ST1926 treatment led to a 30% decrease in the relative expression of Tax mRNA in spleen leukemic cells (Figure 5B). Consistent with the mRNA decrease, we observed a reduction of 40% Tax DNA in treated animals (Figure 5C). Thus, ST1926 induces apoptosis and decreases Tax expressing cells in ATL mice.
Discussion
Here we report that pharmacologically achievable concentrations of ST1926 induce growth inhibition and massive apoptosis in malignant T cells and primary ATL cells with no effect on normal lymphocytes. This ST1926 effect was more pronounced than other synthetic retinoids such as HPR17 and CD437 (supplemental Figure 1), in particular in the HTLV-1–positive cells. ST1926 has the same crucial structural and pharmacological groups as CD437, namely the hydroxyl group, the lipophilic adamantyl moiety, and the carboxylic function. However, ST1926 was designed to have a styrene moiety replacing the naphthalene ring of CD437, resulting in a pharmacokinetically stable, highly orally bioavailable, and pharmacologically attainable in the plasma of patients at micromolar concentrations.23,24 ST1926 is a potent inducer of DNA damage and apoptosis.
HTLV-1–positive cells are very sensitive to ST1926, which may be due to ST1926-induced early reduction of Tax oncoprotein levels, whereas the other synthetic retinoids do not exert this effect17 (Figure 3F). However, the molecular mechanisms by which ST1926 reduces Tax protein levels remain to be elucidated. Recent studies have indicated a role of the proteasome in mediating the ST1926 apoptotic response in acute myeloid leukemia cells,33 reminiscent of Tax proteasome degradation induced by the combination of As and IFN.40,41
In addition to Tax downregulation, we show that ST1926 causes caspase activation and partially caspase-dependent cell death in malignant T cells, as previously reported for several other cancer cell types.33,42 HTLV-1–transformed cells possess in general a wild-type p53; however, the oncoprotein Tax can inhibit p53 signaling by several mechanisms involving phosphorylation of different serine residues and requiring nuclear factor-κB activation among others.43-45 Despite ST1926-induced increase in p53 levels, growth inhibition was p53-independent as Jurkat cells, which have a mutant p53, and Molt-4E6 cells transfected with the human papillomavirus E6, which inhibits p53 function through direct binding,46 are both still responsive to ST1926. Finally, we observed early induction of γ-H2AX implicating ST1926 in eliciting DNA damage in treated malignant T cells as previously reported in other cancer models.23,26,33,34,47 Although CD437 is also an inducer of DNA damage, ST1926 was shown to be more potent in a wide range of DNA lesions, including strand breaks, incomplete excision repair, and alkali-labile sites.26
In vivo, ST1926 significantly prolonged survival in ATL mice (Figure 4A-B) and clearly reduced micro-metastases within the liver parenchyma, as well as in the kidneys and lungs (Figure 4E; data not shown). Although the spleen was the least improving organ after ST1926 treatment, ATL spleen-derived cells showed clear evidence of apoptosis as indicated by PARP cleavage (Figure 5A). Furthermore, downregulation of Tax expression and upregulation of p53 and p21 protein levels following ST1926 treatment suggest a reactivation of p53 signaling in vivo to either restore apoptosis or reactivate senescence of dormant leukemic cells, a key process observed in APL following oncoprotein degradation.48 The level of decrease in Tax mRNA and Tax DNA is quite similar, suggesting that the decrease in Tax expression is the result of decreased number of murine ATL cells following ST1926 treatment in vivo. It is important to note that Tax and HTLV-1 bZIP factor (HBZ) are critical for T-cell lymphoma arising from HTLV-1 infection.49 HBZ is transcribed in all ATL cases.50 It would be interesting in future studies to determine the efficacy of ST1926 on HBZ expression in ATL leukemogenesis. Interestingly, ST1926 was effective in our murine ATL derived from Tax transgenics; however, these cells do not express HBZ.
Finally, we previously reported the remarkable efficacy of the combination of As and IFN in vitro40,51 and in vivo.29 This combination cured Tax-driven murine ATL through specific targeting of leukemia initiating cells secondary to Tax degradation.29 Hence, combining ST1926 to As and IFN may show a greater antileukemic activity, thus fastening leukemia initiating cell clearance in vivo.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Zeinab Sweidan, Nadim Tawil, and Lea Maalouf for technical assistance. The authors are grateful to the personnel of the Core Laboratory Facilities and the Medical Laboratories and the Animal Care Facility of the American University of Beirut.
This work was supported by the Lebanese National Council for Scientific Research, the American University of Beirut Medical Practice Plan, and the American University of Beirut University Research Board.
Authorship
Contribution: H.E.H., R.N., W.W.H., H.H., G.Z., G.D., C.P., A.B., and N.D. designed the study; H.E.H., B.K., B.G., S.S., R.A.-S., A.S., A.G., and M.J. performed experiments; H.E.H., A.B., and N.D. wrote the manuscript; A.G. performed statistical analysis; H.E.H., A.B., and N.D. analyzed data; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: C.P. was a full-time employee of Sigma-Tau Industrie Farmaceutiche Riunite SPA, Pomezia (Roma), Italy, which provided CD437 and ST1926. He is currently a full-time employee of Biogem, Research Institute, Ariano Irpino, Italy, which also supplied ST1926. The remaining authors declare no competing financial interests.
Correspondence: Nadine Darwiche, Department of Biochemistry and Molecular Genetics, American University of Beirut, Beirut/Lebanon, PO Box 11-0236, Beirut, Lebanon; e-mail: nd03@aub.edu.lb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal