Key Points
Physiological shear induces membrane scrambling and microvesiculation in agonist-stimulated platelets.
Rac1 plays a general role in the platelet procoagulant response to shear and is important for coagulation in vitro and in vivo.
Abstract
Activated platelets facilitate blood coagulation by exposing phosphatidylserine (PS) and releasing microvesicles (MVs). However, the potent physiological agonists thrombin and collagen poorly induce PS exposure when a single agonist is used. To obtain a greater procoagulant response, thrombin is commonly used in combination with glycoprotein VI agonists. However, even under these conditions, only a percentage of platelets express procoagulant activity. To date, it remains unclear why platelets poorly expose PS even when stimulated with multiple agonists and what the signaling pathways are of soluble agonist-induced platelet procoagulant activity. Here we show that physiological levels of shear present in blood significantly enhance agonist-induced platelet PS exposure and MV release, enabling low doses of a single agonist to induce full-scale platelet procoagulant activity. PS exposed on the platelet surface was immediately released as MVs, revealing a tight coupling between the 2 processes under shear. Using platelet-specific Rac1−/− mice, we discovered that Rac1 plays a common role in mediating the low-dose agonist-induced procoagulant response independent of platelet aggregation, secretion, and the apoptosis pathway. Platelet-specific Rac1 function was not only important for coagulation in vitro but also for fibrin accumulation in vivo following laser-induced arteriolar injury.
Introduction
Platelets facilitate blood coagulation by externalizing phosphatidylserine (PS) and releasing procoagulant microvesicles (MVs). This function is referred to as platelet procoagulant activity (PPA).1 PS exposure and microvesiculation are assumed to be Ca2+-dependent events, because both are elicited by Ca2+ ionophores,2-4 the endomembrane Ca2+ adenosine-5′-triphosphate (ATP)ase inhibitor thapsigargin,5,6 and the complement membrane attack complex C5b-9.7,8 However, physiological platelet agonists alone, even the potent platelet agonist thrombin or collagen, are very weak in inducing PPA compared with Ca2+-mobilizing agents.9,10 Thus, a combination of thrombin and collagen (or other glycoprotein VI agonists) has to be used to achieve a more observable procoagulant response from platelets.2,3,10-13 It is thus presumed that platelets only express high coagulation-promoting activity when multiple receptor pathways become activated.13,14 Based on these observations, it was proposed that only a percentage of collagen and thrombin-activated platelets express PPA.15
Platelets circulate in the vasculature under shear stress, which normally do not induce procoagulant activity. To this extent, previous work focused on studying agonist-induced PPA under static conditions or von Willebrand factor (VWF)-induced PPA under pathological levels of shear stress that involve the interaction between VWF and glycoprotein Ib-IX (GPIb-IX),16-18 which mediates platelet activation via a rather unique GPIb-IX–dependent signaling pathway.19-23 It was also shown that extremely high shear stress is sufficient to induce platelet microvesiculation (40 000 seconds−1) independent of platelet agonists.24 We asked the question: are physiological levels of shear stress in blood flow involved in PPA induced by GPIb-IX–independent platelet agonists?
Here we show that thrombin and collagen, although unable to induce significant PPA under static conditions, induce high levels of PS exposure and microvesiculation under physiological levels of shear stress. Increasing levels of shear are associated with progressively increased agonist-induced PS exposure and microvesiculation in platelet suspensions even at low agonist concentrations not previously shown to induce PPA. Furthermore, the PS exposed is mostly released as PS-exposed MVs under shear stress. We further demonstrate that Rac1, a member of the Rho family of small GTPases, serves as an important signaling mechanism mediating low-dose agonist-induced PPA and plays an important role in promoting coagulation in vitro and in vivo.
Methods
Generation of mice with Rac1−/− platelets
Animal use and protocols were approved by the Institutional Animal Care Committee of the University of Illinois at Chicago. Megakaryocyte- and platelet-specific conditional Rac1−/− mice were generated as recently described.19
Preparation of human and mouse platelets
For human subjects, Institutional Review Board approval was obtained from the University of Illinois at Chicago, and informed consent was provided according to the Declaration of Helsinki. Human and murine platelets were prepared as previously described.25
Flow cytometric analysis of PS exposure and MV release
For detailed procedures, please see supplemental Data available on the Blood Web site. Briefly, washed platelets were stimulated with thrombin, collagen, or A23187 for 8 minutes. ABT-737 (Calbiochem)-induced Annexin V binding was performed as previously described.26 Shear rates between 250 and 6000 seconds−1 was applied for 8 minutes using a clone-plate rheometer (Thermo Scientific Haake), and exposed PS was detected using Alexa Fluor-488–conjugated Annexin V (Invitrogen) according to the manufacturer’s specifications. Samples were immediately analyzed using flow cytometry. MVs and platelets were distinguished according to their light scattering pattern relative to size standard beads (International Society on Thrombosis and Haemostasis [ISTH] standard).27,28
Platelet aggregation and detection of secreted platelet ATP
Clotting (recalcification time)
For detailed procedures, please see supplemental Data. Briefly, clotting of citrated platelet-rich plasma (PRP) was monitored in a cone-plate rheometer (Thermo Scientific Haake) by measuring viscosity in real time or in a turbidimetric lumi-aggregometer (Chrono-Log) after addition of CaCl2.
Imaging of shear-dependent Annexin V binding to thrombi in vitro
For information on imaging, please see supplemental Data.
Fluorescence intravital microscopy
Briefly, platelet and fibrin accumulation was visualized microscopically in wild-type (WT) or platelet-specific Rac1−/− mice following laser-induced injury to cremaster arterioles using DyLight 649-labeled anti-mouse CD42c and Alexa Fluor 488–conjugated anti-fibrin antibodies (59D8).31 For more information, please see supplemental Data.
Statistical analysis
Statistical analysis was performed in GraphPad Prism 5. For parametric data, comparison between 2 groups was performed using the Student t test. One-way analysis of variance and Bonferroni post-test were used to compare >2 groups. The Kruskall-Wallis test and Dunn’s multiple comparison test were used to compare intravital microscopy data.
Results
Shear significantly enhances agonist-induced platelet PS exposure and microvesiculation
Previous reports have shown that physiological agonists, such as thrombin and collagen, are weak inducers of PPA under static conditions.2,3,9,11,13 As platelets normally flow in blood vessels under shear stress, we studied platelet PS exposure induced by these agonists under defined flow shear rates in comparison with that under static conditions. We detected a very small number of PS-exposed events when platelets were activated by thrombin or collagen under static conditions (Figure 1A-B). Low numbers of PS-exposed events were observed even with the combination of thrombin and collagen, although more than platelets stimulated with a single agonist (Figure 1A-B). In contrast, we observed a dramatic increase in PS exposure in response to physiological agonists when platelets were subjected to shear (6000 seconds−1) using a cone-plate rheometer. In fact, the PS-positive events reached >90% when platelets stimulated with thrombin + collagen were subjected to shear. Moreover, shear alone did not induce PS exposure under these conditions in the absence of agonists. Our results thus indicate that shear significantly enhances physiological agonist-induced PS exposure.
The role of shear in agonist-induced PS exposure and microvesiculation in platelets. (A) Typical flow cytometry histograms of washed mouse and human platelets stimulated with thrombin (mouse, 0.05 U/mL; human, 1 U/mL), collagen (mouse, 2.5 µg/mL; human, 6 µg/mL), or thrombin plus collagen, subjected to shear (6000 seconds−1) or not, and stained with fluorescent Annexin V. (B) Quantification of A (mean ± standard error of the mean [SEM], n = 3 for mouse; n = 6 for human). (C-D) Flow cytometry analyses of platelets treated with or without thrombin (mouse, 0.2 U/mL; human, 10 U/mL), subjected to shear and labeled with fluorescent Annexin V; 0.5- (black arrow) and 0.9-µm (gray arrow) standard beads mark particle sizes, and blue lines passing through the size standards define the region between 0.5 and 0.9 µm. (C) SSC vs FSC. (D) FSC vs Annexin V fluorescence. MVs are defined as having sizes below 1 µm in diameter by ISTH consensus. (E) Washed mouse (n = 3) and human (n = 4) platelets were stimulated with 0.1 and 1 U/mL thrombin, respectively, or unstimulated, and subjected to increasing levels of shear. Annexin V binding, and MVs were analyzed using flow cytometry (mean ± SEM). (F-G) Resting or A23187 (100 nM)-stimulated mouse platelets were subjected to shear or not and analyzed for Annexin V binding and MV release. (F) Typical SSC vs FSC density plots and histogram of Annexin V binding. (G) Quantification of Annexin V binding and microvesiculation (mean ± SEM, n = 3). For all data, * and *** represent statistical significance of P < .05 and .001, respectively.
The role of shear in agonist-induced PS exposure and microvesiculation in platelets. (A) Typical flow cytometry histograms of washed mouse and human platelets stimulated with thrombin (mouse, 0.05 U/mL; human, 1 U/mL), collagen (mouse, 2.5 µg/mL; human, 6 µg/mL), or thrombin plus collagen, subjected to shear (6000 seconds−1) or not, and stained with fluorescent Annexin V. (B) Quantification of A (mean ± standard error of the mean [SEM], n = 3 for mouse; n = 6 for human). (C-D) Flow cytometry analyses of platelets treated with or without thrombin (mouse, 0.2 U/mL; human, 10 U/mL), subjected to shear and labeled with fluorescent Annexin V; 0.5- (black arrow) and 0.9-µm (gray arrow) standard beads mark particle sizes, and blue lines passing through the size standards define the region between 0.5 and 0.9 µm. (C) SSC vs FSC. (D) FSC vs Annexin V fluorescence. MVs are defined as having sizes below 1 µm in diameter by ISTH consensus. (E) Washed mouse (n = 3) and human (n = 4) platelets were stimulated with 0.1 and 1 U/mL thrombin, respectively, or unstimulated, and subjected to increasing levels of shear. Annexin V binding, and MVs were analyzed using flow cytometry (mean ± SEM). (F-G) Resting or A23187 (100 nM)-stimulated mouse platelets were subjected to shear or not and analyzed for Annexin V binding and MV release. (F) Typical SSC vs FSC density plots and histogram of Annexin V binding. (G) Quantification of Annexin V binding and microvesiculation (mean ± SEM, n = 3). For all data, * and *** represent statistical significance of P < .05 and .001, respectively.
To determine whether agonist-stimulated platelets also release MVs (<1 µm in diameter as defined by the ISTH standard28 ) under shear stress, we evaluated the size of MVs in platelet suspension relative to 0.5- and 0.9-μm-diameter fluorescent beads (Figure 1C-D). Shear stress (even at 6000 seconds−1) alone did not induce platelet microvesiculation. Thrombin stimulation alone induced the release of small amounts of MVs When platelets were stimulated with thrombin and subjected to shear, however, a dramatic increase in microvesiculation was observed, which was associated with high levels of exposed PS (Figure 1C-D).
We further characterized the relationship between shear and the induction of PS exposure and microvesiculation in agonist-stimulated platelets. Washed mouse or human platelets were subjected to no shear or increasing levels of shear in the presence or absence of thrombin. Less than 5% of platelets showed PS exposure and microvesiculation when stimulated with thrombin in the absence of shear or when subjected to shear without thrombin. In contrast, as little as 250 seconds−1 shear led to a three- and fivefold increase in PS exposure and microsvesiculation, respectively, in thrombin-stimulated human platelets. In mouse platelets, a 9-fold increase in PS exposure and 11-fold increase in MV release were induced by thrombin at 1000 seconds−1 shear. Further increases in the levels of shear led to progressively increased PS exposure and microvesiculation in thrombin-stimulated platelets (Figure 1E). Thus, although thrombin induces Ca2+ mobilization and platelet activation under static conditions,32 it induces minimal PPA without shear. These results indicate that shear is a requirement for PS exposure and microvesiculation to occur in platelets stimulated by physiological agonists and that agonist-induced PPA is shear level dependently increased.
Shear-dependent PS exposure and microvesiculation is a common response to a variety of platelet activation pathways, as the shear dependence is similar in platelets responding to thrombin and collagen or protease-activated receptor 4 agonist peptide (PAR4AP) (supplemental Figure 1). Shear also similarly enhanced PS exposure in platelets adherent to physiological integrin ligand fibrinogen, in the presence of a soluble agonist (supplemental Figure 2). Furthermore, although A23187 can induce microvesiculation and PS exposure in the absence of shear, shear significantly amplified A23187-induced microvesiculation and PS exposure (Figure 1F-G). Together, these data indicate that shear is a critical common mechanism that promotes PS exposure and microvesiculation in activated platelets.
Shear-induced PS exposure stimulated by platelet agonists is coupled with the release of PS-exposed microvesicles
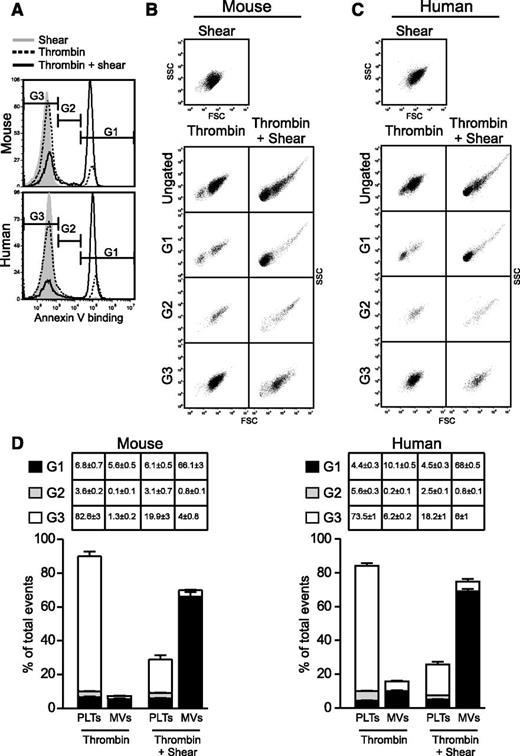
To determine the relationship between PS exposure and microvesiculation under shear, we analyzed the particle size of fluorescent Annexin V binding events in platelets stimulated with thrombin and shear (Figure 2A). Events with high, low, or no Annexin V binding were gated in G1, G2, and G3, respectively, and then plotted in side scatter (SSC) vs forward scatter (FSC) density plots to evaluate their respective size and density profile (Figure 2B-C). This enabled us to evaluate the contribution of platelets and MVs to the total pool of exposed PS detected under shear. Interestingly, the vast majority of the highly fluorescent PS-positive events (gate G1) was associated with MVs, which was dramatically increased following thrombin/shear stimulation (Figure 2B-D). In contrast, the majority of the events that had little to no exposed PS (gate G3) (Figure 2B-D) were platelets. Events with intermediate fluorescence (gate G2) (Figure 2B-D) were a mixture of platelets and aggregates. Therefore, MVs generated under shear have higher levels of exposed PS than activated platelets. These results suggest that once PS is exposed on the surface of agonist-stimulated platelets under shear, it is released as PS-exposed MVs
Shear-dependent PS exposure and microvesiculation are tightly coupled. Thrombin-stimulated mouse (0.2 U/mL) and human (10 U/mL) platelets were subjected to shear or thrombin alone or thrombin + shear and analyzed for PS exposure (Annexin V binding) and MV release using flow cytometry. (A) Histograms showing Annexin V binding and gating of events with high (G1), intermediate (G2), and baseline (G3) fluorescence intensity. (B-C) SSC vs FSC density plots showing the light scatter characteristics of the ungated and gated events from either (B) mouse or (C) human platelets. (D) Quantification of platelets and MVs that were within G1, G2, and G3 gates (mean ± SEM, n = 3 for mouse, n = 5 for human).
Shear-dependent PS exposure and microvesiculation are tightly coupled. Thrombin-stimulated mouse (0.2 U/mL) and human (10 U/mL) platelets were subjected to shear or thrombin alone or thrombin + shear and analyzed for PS exposure (Annexin V binding) and MV release using flow cytometry. (A) Histograms showing Annexin V binding and gating of events with high (G1), intermediate (G2), and baseline (G3) fluorescence intensity. (B-C) SSC vs FSC density plots showing the light scatter characteristics of the ungated and gated events from either (B) mouse or (C) human platelets. (D) Quantification of platelets and MVs that were within G1, G2, and G3 gates (mean ± SEM, n = 3 for mouse, n = 5 for human).
Shear-induced PS exposure and microvesiculation are mediated via Rac1 in activated platelets
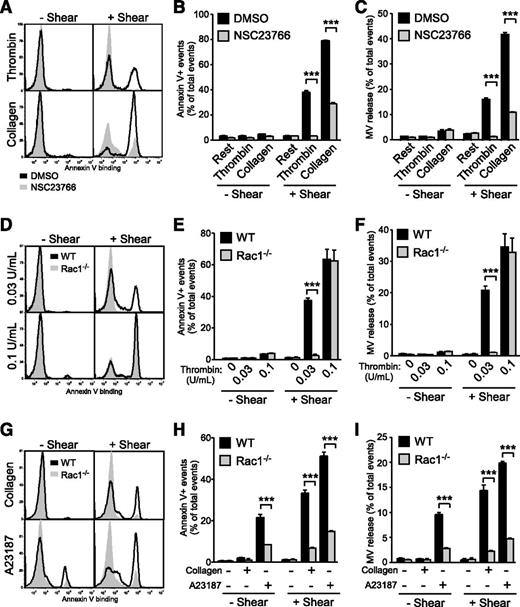
During our investigation into the molecular mechanisms mediating shear-dependent PS exposure and microvesiculation in platelets, we discovered that NSC23766, a small molecule inhibitor of Rac1, inhibits shear-dependent microvesiculation and PS exposure induced by thrombin and collagen (Figure 3A-C). To specifically study the role of platelet Rac1 in shear-induced PPA, we generated mice that lack expression of Rac1 only in megakaryocytes and platelets using the PF4-Cre transgene and the floxed Rac1 allele.19 Indeed, shear-induced PS exposure and microvesiculation was nearly abolished in Rac1−/− platelets stimulated with thrombin or collagen compared with WT (Figure 3D-I). These data demonstrate that Rac1 is critical for shear-induced PS exposure and microvesiculation in platelets stimulated with physiological agonists. Interestingly, this defect was not limited to physiological agonists, but was also observed with A23187 (Figure 3G-I). This is consistent with the above-demonstrated general role of shear in enhancing PS exposure and microvesiculation and suggests that Rac1 mediates a shear-dependent common platelet microvesiculation and PS exposure pathway. High concentrations of agonists, however, rescued the defects of Rac1−/− platelets in microvesiculation and PS exposure, indicating the presence of Rac1-independent signaling pathways under these conditions (Figure 3D-F).
Flow cytometry analysis of the importance of Rac1 in shear-induced PS exposure and microvesiculation in activated platelets. (A-C) Washed human platelets (n = 3) preincubated with 0.1% DMSO or 100 µM NSC23766 were treated with or without 0.1 U/mL thrombin and 5 µg/mL collagen, in the absence or presence of shear. The samples were analyzed for Annexin V binding and MVs using flow cytometry. (A) Histograms and quantification of (B) Annexin V binding and (C) MV release are shown (mean ± SEM, n = 3). (D-F) Washed WT and Rac1−/− platelets were treated with or without 0.03 or 0.1 U/mL thrombin and with or without shear. (D) Histograms of Annexin V binding. (E) Quantification of PS exposure. (F) Quantification of MV release. (E-F, mean ± SEM, n = 4). (G-I) Washed WT and Rac1−/− platelets were treated with or without 2 μg/mL collagen and 50 nM A23187, in the absence or presence of shear (n = 8 and 4, respectively). (G) Histograms of Annexin V binding. (H) Quantification of Annexin V binding. (I) Quantification of MV release (H-I, mean ± SEM). For all data, *** represents statistical significance of P < .001.
Flow cytometry analysis of the importance of Rac1 in shear-induced PS exposure and microvesiculation in activated platelets. (A-C) Washed human platelets (n = 3) preincubated with 0.1% DMSO or 100 µM NSC23766 were treated with or without 0.1 U/mL thrombin and 5 µg/mL collagen, in the absence or presence of shear. The samples were analyzed for Annexin V binding and MVs using flow cytometry. (A) Histograms and quantification of (B) Annexin V binding and (C) MV release are shown (mean ± SEM, n = 3). (D-F) Washed WT and Rac1−/− platelets were treated with or without 0.03 or 0.1 U/mL thrombin and with or without shear. (D) Histograms of Annexin V binding. (E) Quantification of PS exposure. (F) Quantification of MV release. (E-F, mean ± SEM, n = 4). (G-I) Washed WT and Rac1−/− platelets were treated with or without 2 μg/mL collagen and 50 nM A23187, in the absence or presence of shear (n = 8 and 4, respectively). (G) Histograms of Annexin V binding. (H) Quantification of Annexin V binding. (I) Quantification of MV release (H-I, mean ± SEM). For all data, *** represents statistical significance of P < .001.
Rac1 regulates shear-induced PS exposure independent of the apoptosis pathway
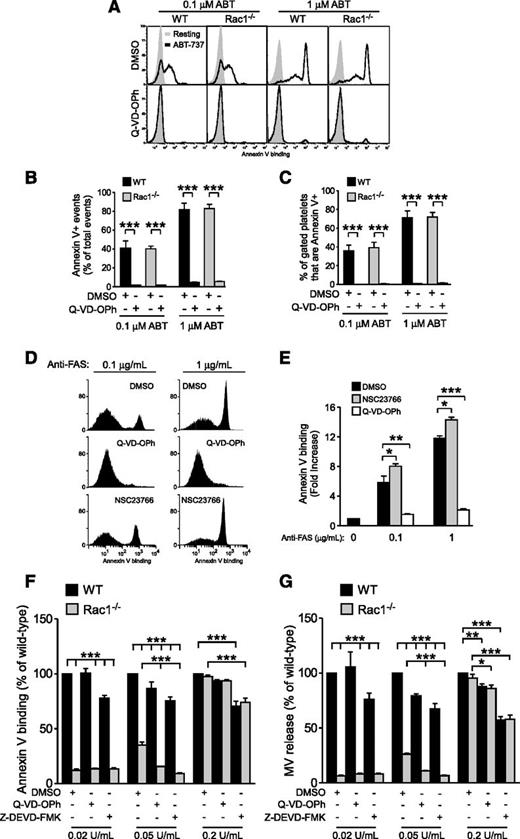
To determine whether Rac1-dependent PS exposure involves the apoptosis signaling pathway, WT and Rac1−/− mouse platelets were stimulated with ABT-737, a BcL-xL inhibitor that promotes Bad/Bax-driven mitochondrial damage, activation of caspases, and caspase-dependent PS exposure,26,33-35 and analyzed via flow cytometry. ABT-737 dose dependently induced PS exposure in both WT and Rac1−/− platelets to the same level (Figure 4A-B), which was abolished by the pan-caspase inhibitor N-(2-Quinolyl)valyl-aspartyl-(2,6-difluorophenoxy)methyl ketone (Q-VD-OPh) (Figure 4A-B), indicating that Rac1 is not required for the PS exposure induced by caspase-dependent apoptosis. Importantly, high levels of PS are predominantly exposed on the platelet surface following ABT-737 treatment (Figure 4C), which is in contrast to the predominant association of externalized PS with MVs when platelets were stimulated with physiological agonists under shear (Figure 2). To further verify that Rac1 is not involved in PS exposure during apoptosis in general, Jurkat cells were treated with vehicle control, NSC23766, or Q-VD-OPh and incubated with anti-FAS (Fas receptor or CD95) to induce extrinsic apoptosis. As expected, anti-FAS–treated Jurkat cells exposed PS in a dose-dependent manner, which was abolished by Q-VD-OPh (Figure 4D-E). Inhibition of Rac1 did not attenuate but enhanced FAS-induced PS exposure (Figure 4D-E). Thus, Rac1 is dispensable for apoptosis-induced PS exposure and plays a distinct role in agonist-induced PS exposure under shear.
Rac1 regulates shear-induced PS exposure independent of caspase-induced PS exposure. (A-C) WT and Rac1−/− platelets were pretreated with 0.1% DMSO or 20 µM Q-VD-OPh, stimulated with 0.1 or 1 µM ABT-737, and analyzed using flow cytometry to detect PS exposure. (A) Representative histograms of Annexin V binding. (B) Quantification of total PS positive events (mean ± SEM, n = 3). (C) Percentage of platelets that bound Annexin V (Annexin V+) (mean ± SEM, n = 3). (D-E) Jurkat cells were treated with 0.1% DMSO, 20 µM Q-VD-OPh, or 100 µM NSC23766, stimulated with 0.1 or 1 µg/mL anti-FAS, and analyzed via flow cytometry for PS exposure. (D) Representative histograms of Annexin V binding. (E) Quantification of Annexin V-positive events (fold increase relative to the baseline; mean ± SEM, n = 7). (F-G) Washed WT and Rac1−/− mouse platelets treated with 0.1% DMSO, 20 µM Q-VD-OPh, or 40 µM Z-DEVD-FMK (in DMSO) were stimulated with increasing doses of thrombin, subjected to shear, and analyzed for (F) PS exposure and (G) MV release (mean ± SEM, n = 4). The response of thrombin-stimulated WT platelets treated with DMSO was defined as 100% for each thrombin dose. For all data, *, **, and *** represent statistical significance of P < .05, .01, and .001, respectively.
Rac1 regulates shear-induced PS exposure independent of caspase-induced PS exposure. (A-C) WT and Rac1−/− platelets were pretreated with 0.1% DMSO or 20 µM Q-VD-OPh, stimulated with 0.1 or 1 µM ABT-737, and analyzed using flow cytometry to detect PS exposure. (A) Representative histograms of Annexin V binding. (B) Quantification of total PS positive events (mean ± SEM, n = 3). (C) Percentage of platelets that bound Annexin V (Annexin V+) (mean ± SEM, n = 3). (D-E) Jurkat cells were treated with 0.1% DMSO, 20 µM Q-VD-OPh, or 100 µM NSC23766, stimulated with 0.1 or 1 µg/mL anti-FAS, and analyzed via flow cytometry for PS exposure. (D) Representative histograms of Annexin V binding. (E) Quantification of Annexin V-positive events (fold increase relative to the baseline; mean ± SEM, n = 7). (F-G) Washed WT and Rac1−/− mouse platelets treated with 0.1% DMSO, 20 µM Q-VD-OPh, or 40 µM Z-DEVD-FMK (in DMSO) were stimulated with increasing doses of thrombin, subjected to shear, and analyzed for (F) PS exposure and (G) MV release (mean ± SEM, n = 4). The response of thrombin-stimulated WT platelets treated with DMSO was defined as 100% for each thrombin dose. For all data, *, **, and *** represent statistical significance of P < .05, .01, and .001, respectively.
Distinct and additive roles of Rac1 and apoptosis signaling in mediating PPA under shear
To further determine whether Rac1 and caspases represent distinct signaling pathways in shear-induced PPA, we directly compared the effects of Rac1 knockout to caspase inhibition. Thus, WT and Rac1−/− platelets were treated with Q-VD-OPh, the caspase-3 inhibitor benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone (Z-DEVD-FMK), or dimethylsulfoxide (DMSO) control, activated with low to high doses of thrombin (0.02 to 0.2 U/mL) and subjected to shear (Figure 4F-G). As described above, low-dose thrombin-induced, shear-dependent PS exposure and microvesiculation was predominantly Rac1 dependent, and the role of caspases was marginal (Figure 4F-G). Interestingly, the marginal inhibitory effect of the caspase inhibitors was retained with increasing thrombin concentrations, whereas the effect of Rac1 knockout was diminished at high thrombin concentrations (Figure 4F-G). At an intermediate concentration of thrombin (0.05 U/mL), PS exposure and MV release in Rac1−/− platelets were ∼20% and 15% of control WT platelets, which was further inhibited by caspase inhibitors to near zero, suggesting that the Rac1 pathway is distinct from the caspase-dependent apoptosis pathway and that these two pathways play additive roles in the process. This was further demonstrated at very high concentrations of thrombin, where microvesiculation and PS exposure were no longer Rac1 dependent, but the partial inhibitory effects of caspase inhibitors remained similar. Together, our data suggest that the Rac1-dependent pathway of microvesiculation and PS exposure is distinct from the apoptosis pathway and specifically responsible for the agonist-stimulated shear-dependent platelet response, whereas the caspase pathway plays a minor role in this response.
Rac1 regulates shear-induced PPA independent of its role in stimulating platelet secretion and aggregation
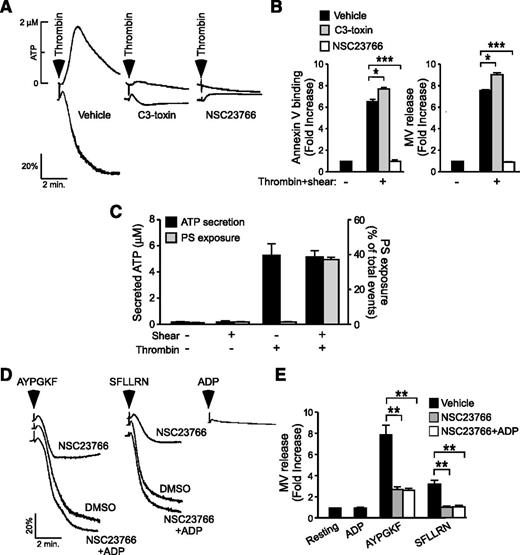
Rac1 was shown to promote platelet secretion and aggregation (supplemental Figure 3).36 Thus, it is possible that the stimulatory effect of Rac1 in the process of shear-dependent PPA is consequential to increases in platelet secretion and aggregation. If this were true, molecules that similarly promote thrombin-induced platelet aggregation should also promote thrombin-induced PPA under shear. Figure 5A shows that C3 toxin, an inhibitor of another ρ GTPase, RhoA, attenuates low-dose thrombin-induced platelet aggregation similar to the Rac1 inhibitor or Rac1−/− platelets.36 This result recapitulates the dose-dependent defect of RhoA−/− platelets in thrombin- and PAR4-induced platelet aggregation.37 In contrast, although inhibition of Rac1 blocked shear-induced PS exposure and microvesiculation in thrombin-stimulated platelets (Figure 5B), inhibition of RhoA had no inhibitory effect (Figure 5B). Rather, C3 toxin significantly enhanced shear-induced PS exposure and microvesculation (Figure 5B). Thus, Rac1 and RhoA similarly up-regulate thrombin-induced platelet secretion and aggregation but play opposing roles in shear-induced PPA, suggesting that platelet aggregation per se is unlikely to be the reason for the role of Rac1. To exclude the possibility that the shear- (and thus Rac1-) dependent PPA is consequential to increased platelet granule secretion, we examined the relationship of shear with granule secretion and PS exposure/microvesiculation. Without platelet agonists, shear alone (up to 6000 seconds−1) neither induces significant granule secretion nor PS exposure/microvesiculation (Figure 5C). Under static conditions, thrombin induced significant granule release, but minimally induced PS exposure. In the presence of shear, however, thrombin induced a dramatic increase in PS exposure although levels of granule secretion were similar to that in the absence of shear. Thus, shear- (and thus Rac1-) dependent platelet PS exposure and microvesiculation is unlikely to be caused by increased granule secretion. Indeed, the inhibitory effect of the Rac1 inhibitor NSC23766 on platelet aggregation was corrected by supplementing the granule content adenosine 5′-diphosphate (ADP; 1 μM) (Figure 5D), indicating that Rac1-dependent ADP secretion is responsible for enhanced platelet aggregation.36 In contrast, supplementation of 1 μM ADP failed to correct the inhibitory effect of NSC23766 on PAR1AP- and PAR4AP-induced microvesiculation under shear (Figure 5E). Thus, the role of Rac1 in promoting shear-induced PPA appears to be independent of its role in promoting platelet secretion and aggregation.
Rac1 regulates shear-induced PS exposure and microvesiculation independent of its role in stimulating platelet secretion and aggregation. (A) Representative aggregation and ATP secretion traces of washed human platelets activated with 0.035 U/mL thrombin following treatment with 2 µg/mL C3-toxin, 100 µM NSC23766, or vehicle control. (B) Washed human platelets were treated with vehicle, 2 µg/mL C3-toxin (C3), or 100 μM NSC23766 (NSC), stimulated with 0.025 U/mL thrombin, subjected to shear, and analyzed for PS exposure and MV release. Data are expressed as mean ± SEM, n = 3. (C) Quantification of ATP secretion (left y-axis) and PS exposure (right y-axis) in washed mouse platelets, either left static or subjected to shear, in the presence or absence of thrombin (0.03 U/mL) (mean ± SEM, n = 3). (D) Aggregation of NSC23766- or DMSO-treated human platelets following stimulation with 60 µM AYPGKF (PAR4) or 2 µM SFLLRN (PAR1) in the presence or absence of 1 µM ADP; 1 µM ADP was added alone as a control. (E) Platelets treated under the same conditions as C were analyzed for the fold increase in shear-induced MV release from the resting baseline (mean ± SEM, n = 8). For all data, *, **, and *** represent statistical significance of P < .05, .01, and .001, respectively.
Rac1 regulates shear-induced PS exposure and microvesiculation independent of its role in stimulating platelet secretion and aggregation. (A) Representative aggregation and ATP secretion traces of washed human platelets activated with 0.035 U/mL thrombin following treatment with 2 µg/mL C3-toxin, 100 µM NSC23766, or vehicle control. (B) Washed human platelets were treated with vehicle, 2 µg/mL C3-toxin (C3), or 100 μM NSC23766 (NSC), stimulated with 0.025 U/mL thrombin, subjected to shear, and analyzed for PS exposure and MV release. Data are expressed as mean ± SEM, n = 3. (C) Quantification of ATP secretion (left y-axis) and PS exposure (right y-axis) in washed mouse platelets, either left static or subjected to shear, in the presence or absence of thrombin (0.03 U/mL) (mean ± SEM, n = 3). (D) Aggregation of NSC23766- or DMSO-treated human platelets following stimulation with 60 µM AYPGKF (PAR4) or 2 µM SFLLRN (PAR1) in the presence or absence of 1 µM ADP; 1 µM ADP was added alone as a control. (E) Platelets treated under the same conditions as C were analyzed for the fold increase in shear-induced MV release from the resting baseline (mean ± SEM, n = 8). For all data, *, **, and *** represent statistical significance of P < .05, .01, and .001, respectively.
Role of platelet Rac1 in coagulation in vitro and in vivo
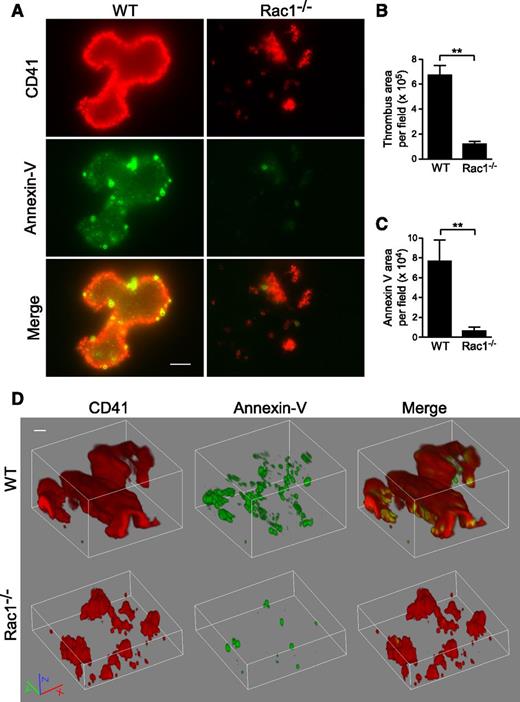
To determine whether Rac1-dependent PS exposure occurs during thrombus formation under shear, WT and Rac1−/− platelets were stimulated with thrombin and subjected to shear over a fibrinogen-coated surface (Figure 6). Indeed, WT platelets formed large thrombi with associated exposed PS, as indicated by Annexin V binding. At high magnification, it is visible that, in addition to significantly increased Annexin V binding that overlaps with WT platelet thrombi, there are numerous small particles and some platelet-like structures with strong Annexin V binding, indicating the presence of PS-positive MVs and platelets (Figure 6A-D). Three-dimensional confocal imaging of thrombi suggests that the strongest PS labeling was mainly located in the lower flanks of a thrombus near the bottom (supplemental Movie 1). Notably, Rac1−/− platelet thrombi showed reduced size and diminished PS exposure (Figure 6A-D; supplemental Movie 2). These data indicate that shear-dependent PS exposure occurs in this in vitro thrombus formation model, and Rac1 is important in the process.
Rac1 is important for the exposure of PS during thrombus formation in vitro under shear. Washed WT and Rac1−/− platelets were stimulated with 0.03 U/mL thrombin and subjected to shear (6000 seconds−1) over a fibrinogen-coated surface. Platelets (red) were stained using anti-mouse CD41 and Alexa Fluor-546–conjugated anti-rat antibodies. Surface-exposed PS (green) was stained using Alexa Fluor-488–conjugated Annexin V. (A) Representative fluorescence microscopy images showing WT and Rac1−/− platelet thrombi (red) and exposed PS associated with thrombi (green). Scale bar represents 10 μm. (B) Quantification of surface area of thrombi per field (mean ± SEM, n = 7). (C) Quantification of the Annexin V surface area per field (mean ± SEM, n = 7). (D) Representative 3-dimensional reconstruction of confocal Z-stack images of WT and Rac1−/− platelet thrombi and exposed PS. Scale bar represents 10 μm. Movies of confocal Z-stack images of WT (supplemental Movie 1) and Rac1−/− (supplemental Movie 2) platelet thrombi and exposed PS are provided. For all data, ** represents statistical significance of P < .01.
Rac1 is important for the exposure of PS during thrombus formation in vitro under shear. Washed WT and Rac1−/− platelets were stimulated with 0.03 U/mL thrombin and subjected to shear (6000 seconds−1) over a fibrinogen-coated surface. Platelets (red) were stained using anti-mouse CD41 and Alexa Fluor-546–conjugated anti-rat antibodies. Surface-exposed PS (green) was stained using Alexa Fluor-488–conjugated Annexin V. (A) Representative fluorescence microscopy images showing WT and Rac1−/− platelet thrombi (red) and exposed PS associated with thrombi (green). Scale bar represents 10 μm. (B) Quantification of surface area of thrombi per field (mean ± SEM, n = 7). (C) Quantification of the Annexin V surface area per field (mean ± SEM, n = 7). (D) Representative 3-dimensional reconstruction of confocal Z-stack images of WT and Rac1−/− platelet thrombi and exposed PS. Scale bar represents 10 μm. Movies of confocal Z-stack images of WT (supplemental Movie 1) and Rac1−/− (supplemental Movie 2) platelet thrombi and exposed PS are provided. For all data, ** represents statistical significance of P < .01.
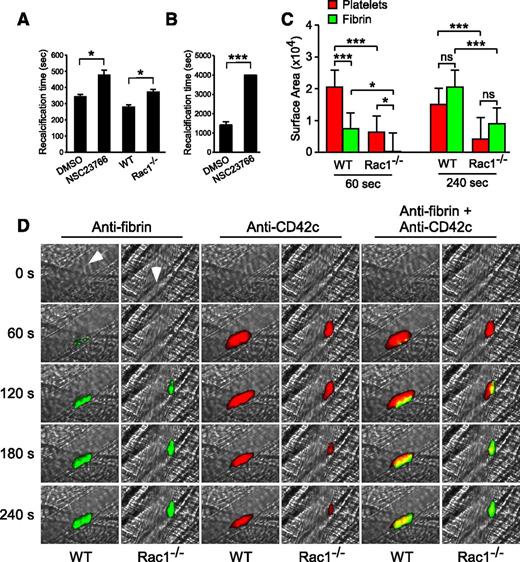
To determine whether the role for Rac1 in stimulating platelet PS exposure and microvesiculation is important to coagulation, we tested the effect of the Rac1 inhibitor (NSC23766) on the clotting time of citrated human PRP induced by adding back CaCl2 (recalcification time) under stirring conditions (Figure 7A), which generate shear, or under defined shear using a cone-plate rheometer (Figure 7B). The Rac1 inhibitor significantly delayed clotting on recalcification (Figure 7A-B). Similarly, platelet-depleted human plasma supplemented with Rac1−/− platelets showed a significant delay in clotting compared with platelet-depleted human plasma supplemented with WT platelets (Figure 7A). These results suggest that Rac1, by stimulating shear-dependent PS exposure and microvesiculation, plays an important role in facilitating coagulation under shear.
Platelet Rac1 is important in promoting fibrin generation in vitro and in vivo. (A) The mean recalcification time ± SEM of human citrated PRP treated with 100 µM NSC23766 (n = 20) or vehicle control (n = 10) and of washed WT and Rac1−/− mouse platelets (n = 5) resuspended in citrated human PPP after addition of CaCl2 under stirring conditions. (B) The recalcification time of human citrated PRP treated with 100 µM NSC23766 or DMSO was monitored using a cone-plate rheometer under defined shear (6000 seconds−1) after addition of CaCl2 (mean ± SEM, n = 4). (C-D) Intravital microscopy was used to monitor fibrin generation (green) and platelet thrombi (red) in vivo following laser-induced cremaster arteriole wall injury in WT and platelet-specific Rac1−/− mice. (C) The sizes of the platelet thrombus and fibrin clot were compared by calculating their respective median surface area at 60 and 240 seconds (median + interquartile range). (D) Representative images of fibrin generation (green) and platelet thrombi formation (red) and merged images. Arrows indicate direction of blood flow. Movies comparing laser-induced platelet thrombus formation and fibrin deposition in WT (supplemental Movie 3) and platelet-specific Rac1−/− mice (supplemental Movie 4) mice, as well as the median integrated fluorescence signals of platelet thrombi and fibrin as a function of time (supplemental Figure 4), are provided. For all data, * and *** represent statistical significance of P < .05 and .001, respectively.
Platelet Rac1 is important in promoting fibrin generation in vitro and in vivo. (A) The mean recalcification time ± SEM of human citrated PRP treated with 100 µM NSC23766 (n = 20) or vehicle control (n = 10) and of washed WT and Rac1−/− mouse platelets (n = 5) resuspended in citrated human PPP after addition of CaCl2 under stirring conditions. (B) The recalcification time of human citrated PRP treated with 100 µM NSC23766 or DMSO was monitored using a cone-plate rheometer under defined shear (6000 seconds−1) after addition of CaCl2 (mean ± SEM, n = 4). (C-D) Intravital microscopy was used to monitor fibrin generation (green) and platelet thrombi (red) in vivo following laser-induced cremaster arteriole wall injury in WT and platelet-specific Rac1−/− mice. (C) The sizes of the platelet thrombus and fibrin clot were compared by calculating their respective median surface area at 60 and 240 seconds (median + interquartile range). (D) Representative images of fibrin generation (green) and platelet thrombi formation (red) and merged images. Arrows indicate direction of blood flow. Movies comparing laser-induced platelet thrombus formation and fibrin deposition in WT (supplemental Movie 3) and platelet-specific Rac1−/− mice (supplemental Movie 4) mice, as well as the median integrated fluorescence signals of platelet thrombi and fibrin as a function of time (supplemental Figure 4), are provided. For all data, * and *** represent statistical significance of P < .05 and .001, respectively.
To investigate whether the role of Rac1 in stimulating shear-induced PPA is important for fibrin formation in vivo, platelet-specific Rac1−/− mice were compared with WT controls in a model of laser-induced cremaster arteriolar thrombosis. WT mice rapidly formed platelet-rich thrombi (Figure 7C-D; supplemental Movie 3) at the site of vascular injury. Initially (60-120 seconds), fibrin was only associated with a small region of the developing platelet thrombus. Later (240 seconds), the platelet thrombi lacking associated fibrin embolized amid the growing fibrin clot, resulting in the overlap and surrounding of platelet thrombi by fibrin (Figure 7C-D; supplemental Movie 3). Platelet-specific Rac1−/− mice were defective in fibrin accumulation (Figure 7C-D; supplemental Movie 4). Although platelet thrombus formation was also reduced in platelet-specific Rac1−/− mice (Figure 7C-D),38 the fibrin clot was much smaller than the platelet thrombus at the early stages of clot formation (60 seconds), indicating that the initial defect in fibrin formation is not the result of a lack of platelet thrombi (Figure 7C: 60 seconds). Later, the platelet thrombi in Rac1−/− mice shrank and were overlapped with and surrounded by the fibrin clot in a manner similar to WT mice (Figure 7C: 240 seconds) but at a much reduced size. Together, these results suggest that platelets promote coagulation in vivo via a Rac1-dependent mechanism and that Rac1-dependent fibrin clot formation is important in stabilizing platelet thrombi and thus determining the ultimate size of stabilized platelet thrombi in vivo.
Discussion
In this study, we identified a mechanism that explains a longstanding enigma that, despite the known importance of activated platelets in facilitating coagulation, physiological platelet agonists (thrombin or collagen) poorly induce a procoagulant response in platelet suspensions.12-14,39 To date, platelets had to be stimulated simultaneously with extremely high doses of multiple platelet agonists to induce marginal levels of PS exposure and microvesiculation. Here we show that platelets become potently procoagulant by releasing MVs with high levels of exposed PS when stimulated with low doses of physiological agonists, but only in the presence of physiological levels of shear found in flowing blood. Although it was previously known that extremely high shear causes platelet microvesiculation and PS exposure during VWF/GPIb-IX–dependent adhesion and aggregation,17,18 physiological levels of shear in normal blood flow clearly do not induce PPA. However, we show that when platelets are activated by common physiological stimuli, this normally innocuous level of shear becomes a major factor that promotes PS exposure and the tightly coupled release of PS-exposed MVs independent of the VWF–GPIb-IX interaction. Shear significantly enhances PPA even at very low concentrations of agonists and increases PS exposure and microvesiculation by an order of magnitude even when platelets are stimulated by the combination of the most potent physiological platelet agonists thrombin and collagen.3 Moreover, shear even enhances A23187-induced PS exposure and microvesiculation. Thus, we introduce a previously unidentified common requirement for shear in platelet PS exposure and microvesiculation.
Another important discovery of this work is that agonist-induced, shear-dependent PPA requires platelet Rac1, as platelet-specific knockout of Rac1 impairs agonist-induced, shear-dependent platelet PS exposure and microvesiculation, leading to a defect in coagulation in vitro and in vivo. Furthermore, the effect of platelet-specific Rac1 knockout excludes the likelihood that NSC23766 nonspecifically attenuates agonist-induced PPA under shear. Rac1-dependent PS exposure is distinct from the known pathway of apoptosis-induced PS exposure. This conclusion is supported by the following evidence. First, shear-dependent PS exposure and microvesiculation is only slightly inhibited by caspase inhibitors, indicating that the capase-dependent apoptotic pathway is only a minor component of shear-dependent PS exposure and microvesiculation induced by platelet agonists, even though these agonists are known to activate the caspase-dependent apoptotic pathway.40 Second, the minor effects of caspase inhibitors and potent effect of Rac1 deficiency are additive. Third, Rac1 deficiency had no effect on apoptosis-mediated PS exposure, suggesting that the apoptotic pathway does not involve Rac1. Importantly, a notable difference between shear/Rac1-dependent and apoptosis-induced PS exposure is that shear/Rac1-dependent externalized PS is predominantly associated with MVs (>95%), indicating a close link between PS exposure and microvesiculation. In contrast, a significant portion of the apoptosis-related PS is associated with platelets or cells (Figure 4). This difference makes sense physiologically, as apoptotic cells expose PS to initiate their own phagocytosis, whereas platelets release procoagulant MVs to maximize the efficiency of coagulation not only on platelet surfaces but also in liquid phase at the site of vascular injury. This major feature of shear-dependent PS exposure is consistent with previous knowledge that platelet-derived MVs have high procoagulant activity3 and explains previous reports suggesting that fibrin formation is not necessarily associated with the site of platelet thrombus formation in vivo.41,42
Several factors that are identified to be associated with platelet PS externalization and MVs thus far have also been known to stimulate platelet granule secretion and aggregation. These include caspases,43 intracellular calcium,5 calcium channels,44 and Rac1, as described here. Thus, it is necessary to determine whether their role in PS exposure and microvesiculation can be secondary to their role in platelet activation.36 Our data indicate that shear/Rac1-dependent platelet PS exposure and MV release is not associated with platelet granule secretion and aggregation. Importantly, supplementation of the granule content ADP to Rac1-inhibited platelets rescued platelet aggregation but failed to rescue shear-induced PPA. Furthermore, shear did not affect thrombin-induced granule secretion but dramatically enhanced PPA. These data suggest that the role of Rac1 in mediating shear-dependent platelet PS exposure and microvesiculation is likely to be independent of its role in promoting platelet secretion and aggregation.36 Importantly, consistent with these in vitro observations, we further showed that the in vivo fibrin clot size in arterial flow is independent of the size of platelet thrombi. In contrast, no matter how large the platelet thrombus size, platelet thrombi are not stable without fibrin deposition. At the later stage in thrombosis, platelet thrombi not associated with the fibrin clot eventually embolize. Thus, the smaller fibrin clot in platelet-specific Rac1−/− mice arterioles results in smaller ultimate platelet thrombi compared with WT. Hence, our data suggest that platelet-dependent Rac1 function facilitates coagulation in vivo and that platelet-associated fibrin helps to stabilize platelet thrombi.
Taken together, we made a significant new finding that shear stress represents a previously unidentified critical factor that is required for PS exposure and microvesiculation stimulated by physiological platelet agonists independent of the VWF–GPIb-IX interaction. Furthermore, we discovered an important role for Rac1 as a novel mediator of shear-dependent PS externalization and the shedding of PS-exposed MVs and in promoting coagulation in vitro and in vivo. These advancements have important general implications to the cardiovascular field, cell biology, and the development of new treatments for hemostatic, thrombotic, and inflammatory disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Hartmut Weiler for kindly providing the anti-fibrin antibody.
This work was supported by National Institutes of Health, National Heart, Lung and Blood Institute (NHLBI) grants HL062350 and HL080264 (to X.D.). M.K.D. was a recipient of a predoctoral fellowship from the American Heart Association Midwest Affiliate.
Authorship
Contribution: M.K.D. performed experiments, analyzed/interpreted data, and prepared the manuscript; J.L., K.K., B.S., and A.S.-T. performed experiments; J.C. prepared the manuscript; Y.Z. provided the mice; and X.D. designed research, analyzed/interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaoping Du, Department of Pharmacology, University of Illinois College of Medicine, 835 South Wolcott Ave, Chicago IL 60612; e-mail: xdu@uic.edu.

![Figure 1. The role of shear in agonist-induced PS exposure and microvesiculation in platelets. (A) Typical flow cytometry histograms of washed mouse and human platelets stimulated with thrombin (mouse, 0.05 U/mL; human, 1 U/mL), collagen (mouse, 2.5 µg/mL; human, 6 µg/mL), or thrombin plus collagen, subjected to shear (6000 seconds−1) or not, and stained with fluorescent Annexin V. (B) Quantification of A (mean ± standard error of the mean [SEM], n = 3 for mouse; n = 6 for human). (C-D) Flow cytometry analyses of platelets treated with or without thrombin (mouse, 0.2 U/mL; human, 10 U/mL), subjected to shear and labeled with fluorescent Annexin V; 0.5- (black arrow) and 0.9-µm (gray arrow) standard beads mark particle sizes, and blue lines passing through the size standards define the region between 0.5 and 0.9 µm. (C) SSC vs FSC. (D) FSC vs Annexin V fluorescence. MVs are defined as having sizes below 1 µm in diameter by ISTH consensus. (E) Washed mouse (n = 3) and human (n = 4) platelets were stimulated with 0.1 and 1 U/mL thrombin, respectively, or unstimulated, and subjected to increasing levels of shear. Annexin V binding, and MVs were analyzed using flow cytometry (mean ± SEM). (F-G) Resting or A23187 (100 nM)-stimulated mouse platelets were subjected to shear or not and analyzed for Annexin V binding and MV release. (F) Typical SSC vs FSC density plots and histogram of Annexin V binding. (G) Quantification of Annexin V binding and microvesiculation (mean ± SEM, n = 3). For all data, * and *** represent statistical significance of P < .05 and .001, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/12/10.1182_blood-2014-03-560821/4/m_1957f1.jpeg?Expires=1769081874&Signature=MkKxWTtscAORTAKrMNhqoHweoHFvBLmc770Jh0p1IZ69VPtr~nLmgDJmD5vn7fcC5eKZydCgfrFgK~7Pta85np71m-MZtunPbu6JG1bG5~W87tCJa6PKSUEO4zvx0kbVASotl~YYTKnK9Q8NA5PCpMHRVEvvYqHwmWL2c-6BOJ94oRUn~M56FQ7wo3vjxuCGZBLC~I4J8uJwYHkALbUojGU7MOKyYdGnqFg13plF7D3Dn99L90f6tRaYE3xYvX08dAtcVLtZPJqz~AEw~yyIxwMgP9q-AWaJucW5-wPpChYt9p9Asmm4rlYn6SgJtMEDVQ6oFrAOzEjR-88qpwCYXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal