Key Points
Bortezomib ameliorates sclerodermatous cGVHD responses by inhibiting germinal center B cells while maintaining GVT effects in murine models.
Bortezomib provides therapeutic benefits for patients with active steroid-refractory cGVHD.
Abstract
Chronic graft-versus-host disease (cGVHD) following allogeneic hematopoietic stem cell transplantation (HSCT) has emerged as a predominant complication following HSCT and has a distinct etiology. We and others have previously demonstrated that bortezomib, a proteasome inhibitor, can prevent but not treat acute GVHD in mice. To assess the effects of bortezomib on cGVHD, a mouse minor histocompatibility antigen-mismatched strain combination was used to mimic clinical cGVHD sclerodermatous pathogenesis and phenotype. Treatment of ongoing cGVHD with bortezomib ameliorated cutaneous lesions, which were also associated with a reduction in total numbers of germinal center B cells and lower B-cell activating factor gene expression levels in cutaneous tissues. Importantly, lymphoma-bearing mice receiving allogeneic HSCT with bortezomib preserved graft-versus-tumor (GVT) effects. Based on these animal studies, we initiated an intrapatient dose escalation clinical trial in patients with extensive steroid–intolerant, dependent, or resistant cGVHD. Marked clinical improvement was observed in patients, which was also associated with reductions of peripheral B cells and minimal toxicity. These results indicate that bortezomib can be of significant use in the treatment of cGVHD and may also allow for maintenance of GVT. This trial was registered at www.clinicaltrials.gov as #NCT01672229.
Introduction
Allogeneic hematopoietic stem cell transplant (HSCT) is currently used for the treatment of a variety of neoplastic hematologic diseases.1 However, development of graft-versus-host disease (GVHD), both acute and chronic, remains a major complication of HSCT, severely limiting its clinical efficacy.2 Furthermore, with the increasing use of reduced intensity and nonablative transplant regimens and the increased age of recipients, the incidence of chronic GVHD (cGVHD) is on the rise, and steroid-refractory cGVHD is now emerging as a predominant cause of morbidity and mortality.3 cGVHD has a distinctive pathogenesis and can manifest with either lichenoid or scleroderma-like cutaneous manifestations of epidermal atrophy, dermal fibrosis, loss of hair follicles, and replacement of peri-eccrine fat.4 It has been demonstrated that B-cell dysregulation with increased output of allo-antibodies, which accumulate within target tissues of the recipient, plays a pivotal role in the pathogenesis of cGVHD seen in both animal models and humans.5-9 Success with second-line therapies is limited in steroid-refractory cGVHD.10 Bortezomib is the first in a class of proteasome inhibitors approved by the Food and Drug Administration for treating multiple myeloma and mantle cell lymphoma patients.11 The anti-inflammatory and direct tumoricidal effects of bortezomib have made it a potential candidate for dissociating graft-versus-tumor (GVT) from GVHD effects. Nevertheless, the diverse effects of bortezomib administrated at different stages of acute GVHD (aGVHD) responses seen in mouse models, ranging from beneficial for aGVHD prevention to detrimental for aGVHD therapy later after HSCT, suggest that incorporation into clinical use merits careful evaluation.12,13 Because aGVHD systems cannot be extrapolated with confidence to the distinct pathophysiological setting of cGVHD, we sought to evaluate the efficacy of bortezomib in murine models of cGVHD with the goal of translating the preclinical data into a human cGVHD clinical trial. Considering the critical role of B-cell dysregulation and allo-antibody production in the pathogenesis of cGVHD and the known inhibitory effects of bortezomib on B cells and plasma cells,14-16 we hypothesized that bortezomib can provide therapeutic benefits against cGVHD through suppression of B-cell or plasma cell responses. We demonstrate that as opposed to aGVHD, bortezomib can be used to successfully treat ongoing cGVHD in mice yet still allow for GVT to occur. Based on the data gleaned from the murine study, we initiated a pilot human clinical trial for the treatment of extensive cutaneous cGVHD resulting in positive clinical responses with minimal toxicity, indicating that bortezomib may offer a treatment option for patients with steroid-refractory cGVHD.
Material and methods
Mice
Eight- to -10 week-old B10.D2 mice were obtained from Jackson Laboratory and 8-to 10-week-old female BALB/c mice were obtained from Taconic Farms. Mice were evaluated by skin clinical scores twice weekly after HSCT and monitored for body weight loss and viability. Bortezomib was dissolved in vehicle (phosphate-buffered saline [PBS]) at the dose indicated in the manuscript and injected intraperitoneally every 5 days after HSCT from day 20, when mice began developing skin cGVHD symptoms. The criteria for euthanasia of survival studies were based on clinical scores and tumor-related symptoms. Skin clinical score were evaluated based on previous studies.17 Mice developing clinical skin scores >3.3 (on a scale of 3.9) or with severe ischemia tail lesions, severe diarrhea, body weight loss, and tumor-related leg paralysis were killed. All mice were maintained at the University of California Davis Medical Center vivarium in accordance with Institutional Animal Care and Use Committee standards.
HSCT
Eight- to 10-week-old BALB/c mice (H2d) received lethal total body irradiation (800 cGy; 137Cs source) and underwent transplantation from donor B10.D2 mice (H2d). Bone marrow ells (8 × 106 cells) with or without spleen cells (25 × 106 cells) were injected intravenously through the tail vein into recipient mice. Mice injected with unfractionated spleen cells were then randomly allocated into different groups for further treatments. Mice were monitored for clinical score, body weight loss, and activities after bone marrow transplantation (BMT) at least twice weekly.
Antibodies and flow cytometry
Skin samples were prepared as previously described.18 The flow cytometry protocols were followed as described previously.12 Briefly, single cell suspensions (1 million cells) were first incubated with Fc Block (BD Pharmingen, San Diego, CA) for 10 minutes and then coincubated with antibodies for 20 minutes at 4°C, followed by washing with staining buffer (PBS + 1% fetal bovine serum). Foxp3 intracellular staining was performed using an eBioscience kit (Cat#00-5523-00) according to the manufacturer’s protocol. Flow cytometry was performed on a LSR Fortessa, and data were analyzed by FlowJo software (TreeStar). CD19-FITC (BD Pharmingen), CD229.1-PE (BD Pharmingen), MHCII-PE-Cy5 (eBioscience), CD4-PE-Cy7 (eBioscience), CD25-APC-Cy7 (BD) FOXP3-FITC (eBioscience), CD45-PB (BioLegend), CD8α-AF700 (BioLegend), B220-PE (BD), IgM-FITC (BioLegend), CD5-AF700 (BioLegend), PNA (BioLegend), CD21-PE (BioLegend), CD23-PE-Cy7(BioLegend), and IgD-PE-Cy7(BioLegend) were used for these studies.
Bioluminescent imaging
A20 (B cell lymphoma) or P815 (mastocytoma) cell lines transfected with luciferase were injected into recipient BALB/c mice intravenously at a dose of 1 × 106 or 6 × 105 cells. Tumor cells were either injected at day 0 or day 20 after BMT as described. Tumor growth was monitored once a week using an IVIS Spectrum imaging system. Briefly, mice were anesthetized by isoflurane and injected with d-luciferin (3 mg/mouse; intraperitoneally). Mice were then imaged 5 minutes after injection with the IVIS Spectrum system. Bioluminescence data were analyzed using Living Image 3.0 (Caliper Life Sciences).
Clinical trial
A single institution, single arm, pilot study of bortezomib in patients with steroid-dependent, -intolerant, or -refractory cGVHD has been initiated and is ongoing at University of California Davis Comprehensive Cancer Center. The clinical trial was approved by the Institutional Review Board of the University of California Davis Medical Center and is in accordance with the Declaration of Helsinki. Informed consent was obtained at enrollment. Patients showing severe single organ (score 3 based on National Institutes of Health [NIH] consensus criteria19 ) or any grade multiorgan (≥3) cGVHD were eligible for enrollment in the study. The study has an intrapatient dose escalation design: patients receive increasing doses of bortezomib starting from 0.2 mg/m2 subcutaneously once a week, and the dose increases every 2 weeks by 0.2 mg/m2 until the patients respond or grade III to IV drug-induced dose-limited toxicity occurs. To avoid unnecessary toxicity, dose escalation was halted in responding patients (defined as 1 level drop in severity score based on NIH consensus criteria) until no further improvement was observed after a minimum of 2 additional doses. Dose escalation was then resumed. Participants were evaluated during enrollment and on days when the bortezomib dosage was increased. Organ-specific cGVHD assessments at involved sites were performed at baseline and at each visit per NIH cGVHD consensus criteria.
Statistics
Weight loss and clinical scores were analyzed by 2-way analysis of variance (ANOVA) with a Tukey post hoc test comparison among groups. Flow cytometry data, bioluminescent imaging, reverse transcriptase-polymerase chain reaction, and pathologic scores were analyzed either by Student t test or 1-way ANOVA with a Tukey post hoc test. P < .05 was considered significant. Survival curves were plotted using a Kaplan-Meier curve and analyzed by log-rank test.
Results
Treatment of mice with active cGVHD with bortezomib results in skin protection
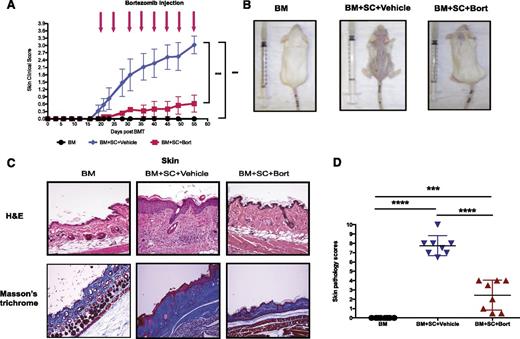
To assess the effects of bortezomib on active cGVHD, a murine model of sclerodermatous cGVHD was established by adoptively transferring unfractionated bone marrow cells with or without spleen cells from donor B10.D2 mice (H2d) into lethally irradiated, major MHC-identical, minor histocompatibility antigen-mismatched recipient BALB/c (H2d) mice. Recipient mice were then treated either with vehicle or bortezomib intraperitoneally at a dose of 0.1 mg/kg on day 20 after transplantation, the point of initiation of clinical symptoms, and every 5 days after to assess the efficacy of bortezomib on treating ongoing cGVHD. In contrast to the vehicle-treated GVHD group, mice receiving bortezomib demonstrated a significant inhibition in the formation of sclerodermatous lesions, with a clinical score of 0.62 ± 0.35 by day 55 as opposed to 3.025 ± 0.36 in the vehicle-treated group (Figure 1A-B; P < .001). Skin pathological assessments revealed that the vehicle group demonstrated typical histopathological features compatible with sclerodermic fibrotic cGVHD (Figure 1C, upper). Most of the lesions showed sclerodermatous changes including epidermal atrophy or irregular acanthosis, dense dermal fibrosis with homogenized and thickened collagen bundles (Figure 1C, lower) with extension into and replacement of perieccrine fat, focal wedge shaped hypergranulosis, loss of hair follicles, and squamatization of the dermal-epidermal junction. Grading of the lesions demonstrated that treatment with bortezomib significantly decreased the cutaneous GVHD pathological score (2.43 ± 0.57 vs 7.75 ± 0.38, P < .0001; Figure 1D).
Therapeutic bortezomib administration protects mice from sclerodermatous cGVHD responses. Irradiated (800 cGy) recipient BALB/c mice received bone marrow cells (8 million) with or without spleen cells (25 million) intravenously from donor B10.D2 mice. Mice were then randomized allocated to treat with either vehicle (PBS) or bortezomib (0.1 mg/kg) intraperitoneally at day 20 after transplant and every 5 days thereafter. (A) Skin clinical scores (on a scale of 3.9) were evaluated twice a week. (B) Photographs were taken at day 55 after HSCT from either bone marrow only or GVHD mice treated with vehicle or bortezomib at day 20. (C) (Upper) Pathologic examination of skin by hematoxylin and eosin stain. (Lower) Collagen deposition and fibrosis were examined by Masson’s trichrome stain. (D) Pathological scores (on a scale of 10) were evaluated by pathologists in a blind code fashion. Data are shown as mean ± standard error of the mean (SEM) and analyzed by 1- or 2-way ANOVA with a Tukey post hoc test to compare between individual groups. *P < .05, **P < .01, and ***P < .001 were considered significant. Data were collected from 2 independent experiments with 8 mice per group.
Therapeutic bortezomib administration protects mice from sclerodermatous cGVHD responses. Irradiated (800 cGy) recipient BALB/c mice received bone marrow cells (8 million) with or without spleen cells (25 million) intravenously from donor B10.D2 mice. Mice were then randomized allocated to treat with either vehicle (PBS) or bortezomib (0.1 mg/kg) intraperitoneally at day 20 after transplant and every 5 days thereafter. (A) Skin clinical scores (on a scale of 3.9) were evaluated twice a week. (B) Photographs were taken at day 55 after HSCT from either bone marrow only or GVHD mice treated with vehicle or bortezomib at day 20. (C) (Upper) Pathologic examination of skin by hematoxylin and eosin stain. (Lower) Collagen deposition and fibrosis were examined by Masson’s trichrome stain. (D) Pathological scores (on a scale of 10) were evaluated by pathologists in a blind code fashion. Data are shown as mean ± standard error of the mean (SEM) and analyzed by 1- or 2-way ANOVA with a Tukey post hoc test to compare between individual groups. *P < .05, **P < .01, and ***P < .001 were considered significant. Data were collected from 2 independent experiments with 8 mice per group.
There were no overt pathological changes in other organs of this murine model (supplemental Figure 1, available on the Blood Web site), except in the gastrointestinal (GI) tract that showed prominent cGVHD characterized by marked fibrosis and collagen deposition. Focal nonspecific acute inflammation seen in the GI tract may be due to residual aGVHD (supplemental Figure 2). Overall, our results indicated bortezomib treatment can successfully ameliorate an ongoing sclerodermatous cGVHD response.
Early therapeutic administration of bortezomib ameliorates scleroderma GVHD response, whereas later administration provides minimal effects
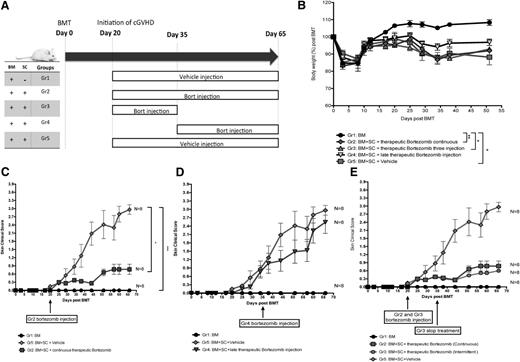
Previously we demonstrated that markedly opposing effects of bortezomib on GVHD outcome were contingent on the timing and dosing of administration but used aGVHD murine model where treatment of ongoing aGVHD resulted in rapid mortality.12 In cGVHD, which has a very different pathophysiology, we observed that bortezomib administration was most efficacious at medium doses (0.1 mg/kg) (supplemental Figure 3A). Interestingly, lower doses of bortezomib reduced the therapeutic effects, whereas higher doses induced significant tissue necrosis in the skin pathology samples (supplemental Figure 3B-E). To further investigate whether differential effects of bortezomib may be contingent on the timing of administration, the administration regimen was divided into early therapeutic regimen (day 20, initiation of skin cGVHD lesions), a late therapeutic regimen (day 35 when cGVHD is well established), and a periodical therapeutic regimen (started at day 20 and stopped at day 35; Figure 2A). There were no obvious changes in body weight loss compared between different administration timing points (Figure 2B). However, administering 0.1 mg/kg bortezomib as an early therapeutic intervention significantly ameliorated the skin lesions (Figure 2C), whereas delayed bortezomib treatment (starting at day 35) in the late phase of skin GVHD pathogenesis did not provide any significant skin therapeutic benefit (Figure 2D). A short course of only 3 intermittent treatments starting at day 20 also provided significant improvement in skin cGVHD scores (Figure 2E), suggesting bortezomib is most efficacious in the early phases of cGVHD pathogenesis. Therefore, the therapeutic window of bortezomib in scleroderma cGVHD is narrow, with medium doses being more efficacious.
Time-dependent administration of bortezomib produces differential scleroderma GVHD responses. Irradiated (8 Gy) BALB/c recipient mice were transplanted with bone marrow cells with or without spleen cells from donor B10.D2 mice. (A) Bortezomib or vehicle administration schema during cGVHD pathogenesis. (B) Body weight changes among different bortezomib regimen groups. (C) Comparison between early therapeutic bortezomib treatments vs vehicle control groups. (D) Comparison between vehicle control groups vs delayed bortezomib treatment groups. (E) Comparison between continuous therapeutic bortezomib treatments vs intermittent therapeutic bortezomib treatment groups. All the groups were tested simultaneously and repeated twice with 8 mice per group. Bortezomib was given at a dose of 0.1 mg/kg for all the groups except bone marrow only and vehicle control groups. The data are shown as mean ± SEM and were analyzed by 2-way ANOVA with a Tukey post hoc test to compare between groups. *P < .05 and ***P < .001 were considered significant.
Time-dependent administration of bortezomib produces differential scleroderma GVHD responses. Irradiated (8 Gy) BALB/c recipient mice were transplanted with bone marrow cells with or without spleen cells from donor B10.D2 mice. (A) Bortezomib or vehicle administration schema during cGVHD pathogenesis. (B) Body weight changes among different bortezomib regimen groups. (C) Comparison between early therapeutic bortezomib treatments vs vehicle control groups. (D) Comparison between vehicle control groups vs delayed bortezomib treatment groups. (E) Comparison between continuous therapeutic bortezomib treatments vs intermittent therapeutic bortezomib treatment groups. All the groups were tested simultaneously and repeated twice with 8 mice per group. Bortezomib was given at a dose of 0.1 mg/kg for all the groups except bone marrow only and vehicle control groups. The data are shown as mean ± SEM and were analyzed by 2-way ANOVA with a Tukey post hoc test to compare between groups. *P < .05 and ***P < .001 were considered significant.
Bortezomib treatment of cGVHD is correlated with lower number of systemic and skin B cells
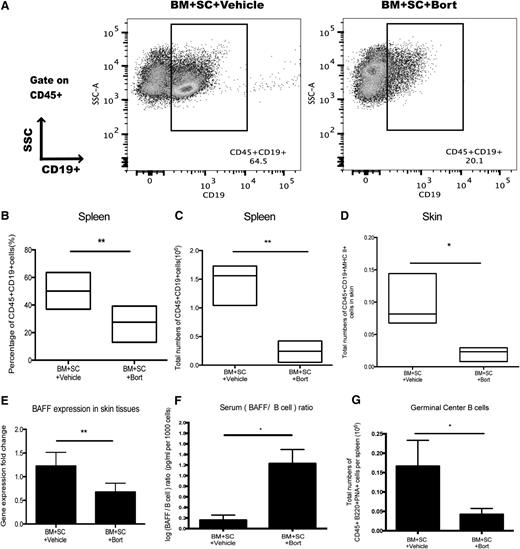
In addition to its effect on plasma cells,15,20 bortezomib has been shown to inhibit activated B cells,14,21 and the pathological significance of B cells and their dysregulation in cGVHD has been well documented in mouse models of cGVHD, as well as correlated in clinical cGVHD.5-8 Furthermore, donor B-cell depletion had been proven to be sufficient to prevent cutaneous GVHD.7,9 Therefore, we hypothesized that bortezomib could suppress B-cell responses, resulting in protection from cGVHD pathogenesis. Activated B cells were shown to undergo apoptosis after bortezomib treatment in vitro (supplemental Figure 4A), and bortezomib was also capable of inhibiting phosphorylated nuclear factor (NF)-κB subunit translocation into the nucleus in B cells (supplemental Figure 4B-E). Further, bortezomib administration resulted in significant reduction of donor derived splenic and skin B cells in vivo (Figure 3A-D). B-cell activating factor (BAFF) expression in the affected skin areas was also decreased in the bortezomib-treated group (Figure 3E). However, BAFF levels were increased in the serum, and a high BAFF/B cell ratio was observed after bortezomib treatment (Figure 3F). To further investigate the effects of bortezomib on donor B-cell reconstitution, we analyzed different subsets of B cells (supplemental Figure 5A-E) and found that bortezomib preferentially targeted germinal center B cells (Figure 3G). However, delayed treatment of bortezomib during cGVHD pathogenesis failed to inhibit germinal center B cells (supplemental Figure 5F), corresponding to the clinical scores (Figure 2D). In addition, dendritic cells were also decreased with bortezomib treatments (supplemental Figure 5G). These results indicate that the treatment of sclerodermatous cGVHD can occur with bortezomib, and the therapeutic effects were associated with reduction in systemic and peripheral B-cell numbers.
B-cell population decreases after bortezomib treatment. Irradiated BALB/c mice transplanted with bone marrow and spleen cells were injected with either bortezomib (0.1 mg/kg) or vehicle control starting at day 20. (A) Spleen cells were collected at day 55 and stained for CD45+CD19+ populations. (B-C) Data showing B-cell populations as percentage and total numbers from spleen. (D) Total numbers of CD45+CD19+MHC II+ B-cell populations in the skin samples harvested on day 55. (E) reverse transcriptase-polymerase chain reaction results detecting BAFF gene expression levels from skin samples collected at day 55. (F) Serum BAFF levels were detected by enzyme-linked immunosorbent assay and calculated as log (BAFF/B cell) ratio. (G) Total numbers of CD45+B220+PNA+ germinal center B cells in the spleen. All the data were collected from 2 independent experiments with 8 mice per group. The data are shown as mean ± SEM and analyzed by Student t test to compare between individual groups. *P < .05 and **P < .01 were considered significant.
B-cell population decreases after bortezomib treatment. Irradiated BALB/c mice transplanted with bone marrow and spleen cells were injected with either bortezomib (0.1 mg/kg) or vehicle control starting at day 20. (A) Spleen cells were collected at day 55 and stained for CD45+CD19+ populations. (B-C) Data showing B-cell populations as percentage and total numbers from spleen. (D) Total numbers of CD45+CD19+MHC II+ B-cell populations in the skin samples harvested on day 55. (E) reverse transcriptase-polymerase chain reaction results detecting BAFF gene expression levels from skin samples collected at day 55. (F) Serum BAFF levels were detected by enzyme-linked immunosorbent assay and calculated as log (BAFF/B cell) ratio. (G) Total numbers of CD45+B220+PNA+ germinal center B cells in the spleen. All the data were collected from 2 independent experiments with 8 mice per group. The data are shown as mean ± SEM and analyzed by Student t test to compare between individual groups. *P < .05 and **P < .01 were considered significant.
Bortezomib promoted donor-derived T-cell reconstitution and skewed T cells toward a regulatory phenotype
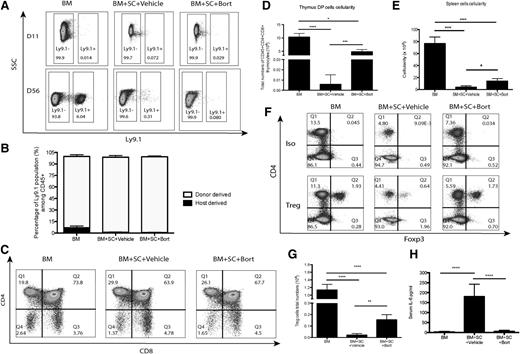
Defects in T-cell reconstitution following BMT may result in possible opportunistic infections and increase the risk of tumor relapse.22,23 Development of cGVHD significantly impairs T-cell reconstitution through thymus damage, and cGVHD patients are reported to be CD4 T-cell lymphopenic.24 To evaluate T-cell reconstitution following HSCT, T-cell subsets were examined among several secondary lymphoid organs. Although bone marrow-only groups demonstrated minimal donor cell chimerism after HSCT, the administration of bortezomib resulted in significantly greater donor chimerism (Figure 4A-B). Treatment with bortezomib also prevented cGVHD-induced loss in thymus cellularity, including the loss of CD4/CD8 double-positive T progenitor cells in the thymus (Figure 4C-D). An increase in donor-derived cells in secondary lymphoid organs was also observed in bortezomib-treated groups (Figure 4E). There were also greater numbers of T-regulatory cells (Tregs) compared with T-conventional (Tcon) cells following bortezomib treatment in the mice with cGVHD (Figure 4F-G). The shift in the Tcon/Treg axis can be explained by the reduction in serum interleukin (IL)-625 observed with bortezomib treatment (Figure 4H). To conclude, bortezomib treatment results in the promotion of thymic recovery and of donor-derived T-cell reconstitution after allogeneic HSCT and also resulted in the skewing of T-cell differentiation toward a Treg phenotype.
T-cell engraftment and Treg cell populations after BMT. To evaluate the effects of bortezomib on T cells, irradiated Balb/c mice were transplanted with bone marrow cells with or without spleen cells and treated with bortezomib (0.1 mg/kg) or vehicle control. Data were collected from 2 to 3 independent experiments with ≥8 mice per group. (A-B) Spleen cells were isolated at either day 11 or day 56 and analyzed for chimerism (Ly9.1+ stain for recipient-derived cells) by flow cytometry. (C-D) Thymus engraftment was analyzed by double-positive T cells (CD4+CD8+) at day 56 by flow cytometry. (E) Cellularity in secondary lymphoid organs at day 56. (F) Spleens were harvested and analyzed for Treg cell populations. (G) Total numbers of Treg cells. (H) Serum IL-6 levels were detected by cytometric bead array. All data were shown as mean ± SEM and analyzed by 1-way ANOVA with a Tukey post hoc test to compare between individual groups. *P < .05, **P < .01, and ***P < .001 were considered significant. Data were collected from 2 independent experiments with 8 mice per group.
T-cell engraftment and Treg cell populations after BMT. To evaluate the effects of bortezomib on T cells, irradiated Balb/c mice were transplanted with bone marrow cells with or without spleen cells and treated with bortezomib (0.1 mg/kg) or vehicle control. Data were collected from 2 to 3 independent experiments with ≥8 mice per group. (A-B) Spleen cells were isolated at either day 11 or day 56 and analyzed for chimerism (Ly9.1+ stain for recipient-derived cells) by flow cytometry. (C-D) Thymus engraftment was analyzed by double-positive T cells (CD4+CD8+) at day 56 by flow cytometry. (E) Cellularity in secondary lymphoid organs at day 56. (F) Spleens were harvested and analyzed for Treg cell populations. (G) Total numbers of Treg cells. (H) Serum IL-6 levels were detected by cytometric bead array. All data were shown as mean ± SEM and analyzed by 1-way ANOVA with a Tukey post hoc test to compare between individual groups. *P < .05, **P < .01, and ***P < .001 were considered significant. Data were collected from 2 independent experiments with 8 mice per group.
Bortezomib ameliorates cGVHD while maintaining GVT effects
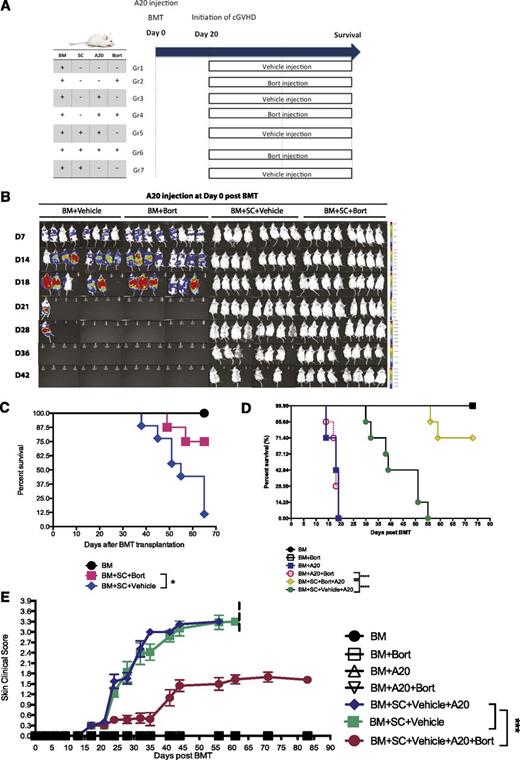
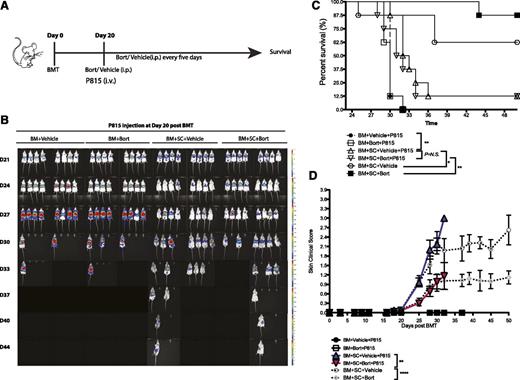
To determine the effects of bortezomib administration on GVT following HSCT, mice were challenged with A20 lymphoma cells at the time of HSCT (Figure 5A). Although mice receiving bone marrow alone succumbed to tumor-related mortality by day 28, mice receiving bone marrow and splenocytes with vehicle or bortezomib at day 20 showed no evidence of tumor outgrowth (Figure 5B). Administration of bortezomib in conjunction with donor spleen cells provided a significant survival benefit both in a cGVHD model (Figure 5C) and a cGVHD/GVT model (Figure 5D) by simultaneously ameliorating cutaneous sclerodermatous cGVHD reactions up to day 83 after BMT (Figure 5E). The observed effect of bortezomib on tumor cells can be partially explained by its direct antitumor effects, because in a HSCT model without spleen cells, bortezomib decreases the tumor burden but does not completely eliminate it when mice were challenged with tumor cells at day 20 (supplemental Figure 6). Bortezomib’s effects on immune parameters were similar with or without tumor challenge (supplemental Figure 7). To further exam the GVT effects, we challenged mice with a more malignant mastocytoma tumor cell line (P815) at day 20 when bortezomib treatments were initiated (Figure 6A). In this high tumor burden challenge, bortezomib was still capable of maintaining GVT effects (Figure 6B-C). Overall, our results demonstrate that bortezomib treatment can successfully ameliorate sclerodermatous cGVHD pathology while maintaining potent GVT effects resulting in significant increases in survival.
Bortezomib administration allows for maintenance of GVT effects while decreasing cGVHD skin lesions in A20 tumor models. Irradiated BALB/c mice were transplanted with bone marrow cells with or without spleen cells at day 0. Six hours later, A20 luciferase-transfected lymphoma cells (1 × 106) were injected through the tail vein into the indicated groups. Bortezomib was administered at day 20 and every 5 days thereafter. (A) Timeline schema for different conditions among groups. (B) Bioluminescent images were acquired to monitor tumor burdens. (C) Survival curves from experimentally treated groups in the cGVHD model. (D) Survival curves from different groups challenged by A20 tumor cell lines at day 0. (E) Skin clinical scores were evaluated twice a week. Data were collected from 1 experiment with 8 mice per group. The data are shown as mean ± SEM and analyzed by 2-way ANOVA with a Tukey post hoc test to compare between individual groups. Survival data were plotted by the Kaplan-Meier method and analyzed by the log-rank test. **P < .01, ***P < .001, and ****P < .0001 were considered significant.
Bortezomib administration allows for maintenance of GVT effects while decreasing cGVHD skin lesions in A20 tumor models. Irradiated BALB/c mice were transplanted with bone marrow cells with or without spleen cells at day 0. Six hours later, A20 luciferase-transfected lymphoma cells (1 × 106) were injected through the tail vein into the indicated groups. Bortezomib was administered at day 20 and every 5 days thereafter. (A) Timeline schema for different conditions among groups. (B) Bioluminescent images were acquired to monitor tumor burdens. (C) Survival curves from experimentally treated groups in the cGVHD model. (D) Survival curves from different groups challenged by A20 tumor cell lines at day 0. (E) Skin clinical scores were evaluated twice a week. Data were collected from 1 experiment with 8 mice per group. The data are shown as mean ± SEM and analyzed by 2-way ANOVA with a Tukey post hoc test to compare between individual groups. Survival data were plotted by the Kaplan-Meier method and analyzed by the log-rank test. **P < .01, ***P < .001, and ****P < .0001 were considered significant.
Bortezomib administration allows for maintenance of GVT effects while decreasing cGVHD skin lesions in P815 tumor models. Irradiated BALB/c mice were transplanted with bone marrow cells with or without spleen cells at day 0. P815-luciferase-transfected mastocytoma cells (6 × 105) were injected through the tail vein into the indicated groups at day 20 when bortezomib treatment was initiated. (A) Timeline schema for different conditions among groups. (B) Bioluminescent images were acquired to monitor tumor burdens. (C) Survival curves from experimentally treated groups. (D) Skin clinical scores were evaluated twice a week. Data were collected from 1 experiment with 8 mice per group. The data are shown as mean ± SEM and analyzed by 2-way ANOVA with a Tukey post hoc test to compare between individual groups. Survival data were plotted by the Kaplan-Meier method and analyzed by the log-rank test. **P < .01, ***P < .001, and ****P < .0001 were considered significant.
Bortezomib administration allows for maintenance of GVT effects while decreasing cGVHD skin lesions in P815 tumor models. Irradiated BALB/c mice were transplanted with bone marrow cells with or without spleen cells at day 0. P815-luciferase-transfected mastocytoma cells (6 × 105) were injected through the tail vein into the indicated groups at day 20 when bortezomib treatment was initiated. (A) Timeline schema for different conditions among groups. (B) Bioluminescent images were acquired to monitor tumor burdens. (C) Survival curves from experimentally treated groups. (D) Skin clinical scores were evaluated twice a week. Data were collected from 1 experiment with 8 mice per group. The data are shown as mean ± SEM and analyzed by 2-way ANOVA with a Tukey post hoc test to compare between individual groups. Survival data were plotted by the Kaplan-Meier method and analyzed by the log-rank test. **P < .01, ***P < .001, and ****P < .0001 were considered significant.
Effects of bortezomib in patients with active steroid-refractory cGVHD
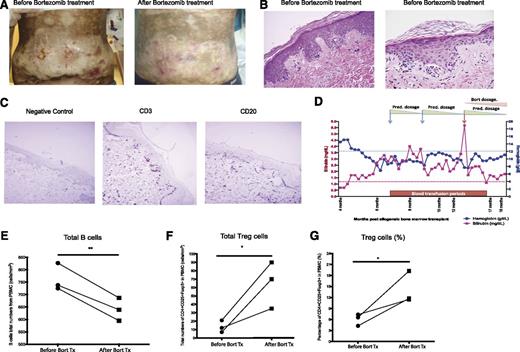
Based on our murine data, we initiated a pilot clinical trial to assess the effect of bortezomib in patients with steroid-refractory, -dependent, or -intolerant severe cGVHD. Treatment options for these patients are extremely limited. Our animal data demonstrated a dose-limiting effect of bortezomib, with minimal therapeutic effects at lower doses and potential toxicity at higher doses (supplemental Figure 3). Therefore, an intrapatient dose escalation design was used to test a wide range of doses safely in each patient. Ten patients have thus far enrolled and either finished the trial or have come off the trial (Table 1). Six of 10 patients received >60% of the scheduled doses. Of these, 5 of 6 patients responded to therapy, achieving partial response defined as a decrease ≥1 point on a 0 to 3-point organ-specific categorical scale per NIH response consensus criteria guidelines. In most of the responders, the dose and/or the number of immunosuppressive drug therapies were reduced during therapy with bortezomib, a measure of efficacy of the drug. Five patients could not finish the scheduled 6 months of therapy (due to a progressive bronchiolitis obliterans and multiple respiratory infections [1], neuropathy [1], viral mouth infection [1], relapse of underlying disease [1], or worsening of thrombocytompenia [1]). Figure 7A-C shows an example of one of the responders. Patient 4 had extensive grade III skin sclerodermatous cGVHD covering >50% of the body (Figure 7A, left). Skin pathology demonstrated typical cGVHD lesions, including homogenized and thickened collagen bundles, representing sclerodermatous features with a high number of T and B cells infiltrated in the skin (Figure 7B-C). At the start of the trial, the patient had multiple nonhealing ulcers and had significant restriction of mobility due to cutaneous cGVHD. Previous therapeutic interventions including intermediate dose steroids and calcineurin inhibitors failed to control the cGVHD lesions. A significant change in patient performance was observed within a few weeks of bortezomib therapy; at higher doses, all the ulcers except for one resolved (Figure 7A, right). The range of motion improved from a score of 3 to 2 based on NIH consensus criteria, and the dose of prednisone was slightly decreased.
Treatment responses of bortezomib on human patients
| Patient number . | Trial start . | Trial stop . | cGVHD organs . | Immunosuppression before trial . | Immunosuppression at conclusion of the study . | cGVHD score before start of the trial . | cGVHD score at conclusion of the study . | Comments . |
|---|---|---|---|---|---|---|---|---|
| 1 | 10/16/12 | 4/10/13 | Steroid refractory ophthalmic GVHD, mouth | Cyclosporine ophthalmic drop | Cyclosporine ophthalmic drop | Eyes 3 | Eyes 1 | Restarted on bortezomib 6 mo after the completion of the study due to relapse of cGVHD. |
| Mouth 1 | Mouth 0 | |||||||
| 2 | 11/20/12 | 1/15/13 | Eyes, mouth, skin | Mycophenolate 100 mg twice daily, tacrolimus 1 mg daily, sirolimus 1 mg twice daily, prednisone 20 mg alternating with 40 mg | Mycophenolate 100 mg twice daily, tacrolimus 1 mg daily, sirolimus 1 mg twice daily, prednisone 20 mg alternating with 40 mg | Mouth 3 | Mouth 3 | Early withdrawal due to mouth sores. |
| Joints 3 | Joints 3 | |||||||
| Skin 2 | Skin 2 | |||||||
| 3 | 01/14/13 | 05/07/13 | Skin, mouth, eyes, GI, lungs, joints, and fascia | Mycophenolate 1000 mg twice daily and prednisone 40 mg qod | Mycophenolate 1000 mg twice daily and prednisone 40 mg qod | 2 for all organs involved | 2 for all organs involved | No significant response was observed |
| 4 | 03/18/13 | 08/27/13 | Skin, GI, mouth, and joint | Tacrolimus 1.5 mg twice daily, mycophenolate 1 g twice daily, prednisone 40 mg/day | Tacrolimus 1.5 mg twice daily, Mycophenolate 1 g twice daily, prednisone 30 mg/day | Skin 3 | Skin 2 | Significant healing of the ulcerated skin and mild softening/ decrease in pigmentation. Improved swallowing |
| GI 2 | GI 1 | |||||||
| Mouth 3 | Mouth 2 | |||||||
| Joint 3 | Joint 2 | |||||||
| 5 | 06/25/13 | 12/10/13 | Steroid-dependent hemolytic anemia, mouth, and eye | Prednisone 60 mg/day and tacrolimus 1 mg a day alternating with 0.5 mg/day | Prednisone 0.5 mg 3 times a week | Anemia: severe | Anemia: mild | Restarted on bortezomib, 4 mo after the completion of the study to maintain the response |
| Mouth 1 | Mouth 0 | |||||||
| Eye 1 | Eye 0 | |||||||
| 6 | 08/02/13 | 09/03/13 | Steroid-refractory skin | Tacrolimus 1.5 mg twice daily | Tacrolimus 1.5 mg twice daily | Skin 3 | Skin 3 | Withdrawn from the study after one month due to relapse of the chronic myelomonocytic leukemia |
| 7 | 08/13/13 | 01/07/14 | Skin, eyes, and mouth | Tacrolimus 0.5 mg thrice daily, Sirolimus 1 mg daily, prednisone 30 mg daily | Tacrolimus 0.5 mg twice daily, prednisone 15 mg alternating with 5 mg daily | Skin 2 | Skin 1 | Responding but withdrawn early from the study due to neuropathy |
| Eyes 2 | Eyes 1 | |||||||
| Mouth 0 | Mouth 0 | |||||||
| 8 | 09/17/13 | 11/05/13 | Skin, mouth, and lung | Prednisone 30 mg daily, sirolimus 1 mg three times a week | Prednisone 15 mg daily alternating with 10 mg a day; tacrolimus 0.5 mg twice a day | Skin 3 | Skin 2 | Withdrawn early from the study due to symptomatic respiratory infections and hospital admissions |
| Mouth 2 | Mouth 1 | |||||||
| Lung 3 | Lung 3 | |||||||
| 9 | 09/20/13 | 03/14/14 | Skin, mouth, eyes, and liver | Cyclosporine 25 mg 3 times a week and prednisone 30 mg daily | None | Mouth 2 | Mouth 0 | Relapse of GVHD 4 mo after the last dose of the study drug |
| Eye 2 | Eye 1 | |||||||
| Skin 1 | Skin 0 | |||||||
| 10 | 12/6/13 | 1/24/14 | Steroid-intolerant and -dependent skin, mouth, and eyes | Prednisone 20 mg qod and cyclosporine 25 mg daily. | Prednisone 20mg qod and cyclosporine 25 mg daily. | Skin 3 | Skin 3 | Withdrawn early from the study due to low platelets |
| Mouth 2 | Mouth 2 |
| Patient number . | Trial start . | Trial stop . | cGVHD organs . | Immunosuppression before trial . | Immunosuppression at conclusion of the study . | cGVHD score before start of the trial . | cGVHD score at conclusion of the study . | Comments . |
|---|---|---|---|---|---|---|---|---|
| 1 | 10/16/12 | 4/10/13 | Steroid refractory ophthalmic GVHD, mouth | Cyclosporine ophthalmic drop | Cyclosporine ophthalmic drop | Eyes 3 | Eyes 1 | Restarted on bortezomib 6 mo after the completion of the study due to relapse of cGVHD. |
| Mouth 1 | Mouth 0 | |||||||
| 2 | 11/20/12 | 1/15/13 | Eyes, mouth, skin | Mycophenolate 100 mg twice daily, tacrolimus 1 mg daily, sirolimus 1 mg twice daily, prednisone 20 mg alternating with 40 mg | Mycophenolate 100 mg twice daily, tacrolimus 1 mg daily, sirolimus 1 mg twice daily, prednisone 20 mg alternating with 40 mg | Mouth 3 | Mouth 3 | Early withdrawal due to mouth sores. |
| Joints 3 | Joints 3 | |||||||
| Skin 2 | Skin 2 | |||||||
| 3 | 01/14/13 | 05/07/13 | Skin, mouth, eyes, GI, lungs, joints, and fascia | Mycophenolate 1000 mg twice daily and prednisone 40 mg qod | Mycophenolate 1000 mg twice daily and prednisone 40 mg qod | 2 for all organs involved | 2 for all organs involved | No significant response was observed |
| 4 | 03/18/13 | 08/27/13 | Skin, GI, mouth, and joint | Tacrolimus 1.5 mg twice daily, mycophenolate 1 g twice daily, prednisone 40 mg/day | Tacrolimus 1.5 mg twice daily, Mycophenolate 1 g twice daily, prednisone 30 mg/day | Skin 3 | Skin 2 | Significant healing of the ulcerated skin and mild softening/ decrease in pigmentation. Improved swallowing |
| GI 2 | GI 1 | |||||||
| Mouth 3 | Mouth 2 | |||||||
| Joint 3 | Joint 2 | |||||||
| 5 | 06/25/13 | 12/10/13 | Steroid-dependent hemolytic anemia, mouth, and eye | Prednisone 60 mg/day and tacrolimus 1 mg a day alternating with 0.5 mg/day | Prednisone 0.5 mg 3 times a week | Anemia: severe | Anemia: mild | Restarted on bortezomib, 4 mo after the completion of the study to maintain the response |
| Mouth 1 | Mouth 0 | |||||||
| Eye 1 | Eye 0 | |||||||
| 6 | 08/02/13 | 09/03/13 | Steroid-refractory skin | Tacrolimus 1.5 mg twice daily | Tacrolimus 1.5 mg twice daily | Skin 3 | Skin 3 | Withdrawn from the study after one month due to relapse of the chronic myelomonocytic leukemia |
| 7 | 08/13/13 | 01/07/14 | Skin, eyes, and mouth | Tacrolimus 0.5 mg thrice daily, Sirolimus 1 mg daily, prednisone 30 mg daily | Tacrolimus 0.5 mg twice daily, prednisone 15 mg alternating with 5 mg daily | Skin 2 | Skin 1 | Responding but withdrawn early from the study due to neuropathy |
| Eyes 2 | Eyes 1 | |||||||
| Mouth 0 | Mouth 0 | |||||||
| 8 | 09/17/13 | 11/05/13 | Skin, mouth, and lung | Prednisone 30 mg daily, sirolimus 1 mg three times a week | Prednisone 15 mg daily alternating with 10 mg a day; tacrolimus 0.5 mg twice a day | Skin 3 | Skin 2 | Withdrawn early from the study due to symptomatic respiratory infections and hospital admissions |
| Mouth 2 | Mouth 1 | |||||||
| Lung 3 | Lung 3 | |||||||
| 9 | 09/20/13 | 03/14/14 | Skin, mouth, eyes, and liver | Cyclosporine 25 mg 3 times a week and prednisone 30 mg daily | None | Mouth 2 | Mouth 0 | Relapse of GVHD 4 mo after the last dose of the study drug |
| Eye 2 | Eye 1 | |||||||
| Skin 1 | Skin 0 | |||||||
| 10 | 12/6/13 | 1/24/14 | Steroid-intolerant and -dependent skin, mouth, and eyes | Prednisone 20 mg qod and cyclosporine 25 mg daily. | Prednisone 20mg qod and cyclosporine 25 mg daily. | Skin 3 | Skin 3 | Withdrawn early from the study due to low platelets |
| Mouth 2 | Mouth 2 |
qod, every other day.
Treatment effects of bortezomib on clinical cGVHD human patients. A single institution pilot study of bortezomib was initiated in patients with steroid-dependent, -intolerant, or -refractory cGVHD. (A) Patient 4 showed extensive grade III skin sclerodermatous GVHD covering >0% of the body. The abdominal region before and after bortezomib treatments are shown. (B) Representative images of the pretreatment skin biopsies taken from the patient shown in A. (C) Immunohistochemical staining for CD3 and CD20 in pretreatment skin biopsy samples from patient 4. (D) CBC and biochemistry data from patient 5 were collected through the trial period. (E) Total numbers of peripheral blood B cells (CD45+CD19+) from 3 patients were analyzed by flow cytometry before and after bortezomib treatment. (F-G) Treg cell populations (CD4+CD25+Foxp3+) were analyzed by flow cytometry and shown as total numbers and percentage. All the data were collected from individual cGVHD patients that underwent bortezomib treatment. The data are shown as mean ± SEM and analyzed by Student t test to compare pre- and postbortezomib treatments. *P < .05 was considered significant.
Treatment effects of bortezomib on clinical cGVHD human patients. A single institution pilot study of bortezomib was initiated in patients with steroid-dependent, -intolerant, or -refractory cGVHD. (A) Patient 4 showed extensive grade III skin sclerodermatous GVHD covering >0% of the body. The abdominal region before and after bortezomib treatments are shown. (B) Representative images of the pretreatment skin biopsies taken from the patient shown in A. (C) Immunohistochemical staining for CD3 and CD20 in pretreatment skin biopsy samples from patient 4. (D) CBC and biochemistry data from patient 5 were collected through the trial period. (E) Total numbers of peripheral blood B cells (CD45+CD19+) from 3 patients were analyzed by flow cytometry before and after bortezomib treatment. (F-G) Treg cell populations (CD4+CD25+Foxp3+) were analyzed by flow cytometry and shown as total numbers and percentage. All the data were collected from individual cGVHD patients that underwent bortezomib treatment. The data are shown as mean ± SEM and analyzed by Student t test to compare pre- and postbortezomib treatments. *P < .05 was considered significant.
Patient 1 was enrolled in the study due to severe debilitating eye cGVHD. The patient had failed multiple therapeutic interventions including several surgical interventions, high-dose systemic and topical steroids, and other immunosuppressive interventions including calcineurin inhibitors. In addition to the eyes, the patient had recurrent ulcers and dryness in the mouth. A response was observed at the second dose level (0.4 mg/m2) with a decrease in the requirement for artificial tears. A significant improvement in the limbal and conjunctival inflammation and a decrease in photophobia were noticed at higher doses, with the severity score decreased from 3 to 1 (NIH consensus criteria). The pain level also decreased from persistent 7 to 8 on a scale of 10 to <2. The patient’s mouth dryness improved, and the patient did not have any further mouth ulcers during the trial period.
Patient 5 developed a significant life-threatening Coombs-negative autoimmune hemolytic anemia starting around 6 months after transplant as a manifestation for cGVHD.19 The patient responded quickly to high-dose steroids (60-100 mg of methylprednisone), but ≥2 separate attempts to taper steroid doses to <30 mg/day resulted in massive hemolysis (Figure 7D). The patient initiated bortezomib therapy; by the end of the study, the patient was off transfusions for 3 months and the prednisone dose was reduced to 5 mg every other day. The hemolysis symptoms were largely improved by several clinical indicators over time (Figure 7D).
Flow cytometry of peripheral blood–derived mononuclear cells from these patients revealed a significant decrease in B cells and an increase in Tregs (Figure 7E-G). These results indicated that, similar to the murine studies, bortezomib administration can be used to treat active cGVHD.
Discussion
Murine models potentially bridge the gap between basic research and human clinical trials. This study demonstrated that the information revealed in the mouse model resulted in similar clinical effects on cGVHD. Patients described in this study are all cases of steroid-dependent/-refractory cGVHD that responded to bortezomib treatment. The majority of the patients had multiple organ involvement and were on several immunosuppressive drugs, with cGVHD scores higher than many similar trials. Nevertheless, we experienced clinically significant improvement reflected either by improvement of cGVHD scores or a decrease in the dose or schedule of anti-GVHD drugs. Importantly, we also observed more subtle but clinically important improvements of cGVHD that were not detected by NIH consensus scoring system such as improvement in skin ulceration. However, despite the clinically significant improvement of inflammation, the fibrotic and hyperpigmentation associated with well-established cGVHD did not resolved in some of the responders, supporting our mouse data on the beneficial effect of early rather than late therapeutic intervention in this patient population. Therefore, using bortezomib as an early therapeutic regimen for cGVHD needs to be further investigated in future clinical trials. Both clinical and murine models resulted in concordant decreases in B cells correlating with responses. Importantly, cGVHD protection did not come at the expense of GVT in the mouse model, but longer assessment is needed to ascertain if similar effects on relapse prevention occur in the clinical study.
The long-term toxicities of any therapeutic intervention for management of chronic diseases such as cGVHD can limit their utilization. With regard to bortezomib’s use after HSCT, higher doses of bortezomib induced skin tissue necrosis in the mouse cGVHD model and early mortality in an aGVHD model (data not shown), whereas medium doses of bortezomib provide significant therapeutic effects, suggesting that the clinical dose of bortezomib merits careful evaluation. Furthermore, the therapeutic effects of bortezomib were also contingent on the timing of administration. In cGVHD, early intervention may achieve better therapeutic effects in sclerodermatous cGVHD responses, whereas later-stage disease treatment may result in less effect. Although early prevention in aGVHD may protect mice from proinflammatory cytokine-mediated gastrointestinal toxicity,26,27 treatment of existing aGVHD may accelerate GVHD-dependent morbidity.12 These results indicate that differential effects of bortezomib are dependent on the pathogenesis of either aGVHD or cGVHD. Furthermore, although we demonstrated the therapeutic effects of bortezomib on sclerodermatous cGHVD response, whether bortezomib can also protect other target organs, such as the bronchiolitis obliterans, needs further investigation.
The pathological significance of B cells in cGVHD is well documented, and a reduction in B cells was observed after bortezomib treatment in both mouse and human patients. Germinal center B cells have been shown to play a significant role in cGVHD pathogenesis,6,28 and our data demonstrated that bortezomib can preferentially target germinal center B cells during B-cell reconstitution. The CD40-CD40L interaction on germinal center B cells can activate persistent canonical NF-κB signaling, which provides survival and proliferation signals to B cells.29 Because bortezomib is an indirect NF-κB inhibitor, it is likely that bortezomib induces germinal center B cells to undergo apoptosis through NF-κB inhibition during reconstitution. BAFF is another important cytokine to regulate B-cell homeostasis, and recent data revealed that elevated BAFF/B-cell ratios can be found in cGVHD patients.5,30 In our data, bortezomib successfully decreased donor-derived B cells and BAFF expression in the skin; however, lower B-cell counts may lead to compensatory increases in BAFF levels in the serum. Although bortezomib had profound effects on B-cells subsets, we cannot rule out that other immune cell subsets or cytokines are also being affected, as IL-17 has also been reported to be important for cutaneous chronic GVHD.31 Therefore, the therapeutic effects of bortezomib may come from the net effects of several immune parameters, including B cells, Treg cells, and dendritic cells.
Although immunosuppressive therapy is effective in most cases of cGVHD, long-term side effects of therapy are particularly deleterious. Furthermore, T-cell dysregulation, a hallmark of GVHD, results in increased incidence of infection, as well as elevated risk of autoimmune disorders. The specifically skewing of Treg/Tcon cells are documented to be potentially important in cGVHD.24 Treg cells were expanded following bortezomib treatment after cGVHD, and this was also associated with cGVHD amelioration in both our mouse and human studies. Although proteasome inhibition is an immunosuppressive intervention, its effect is limited to activated T cells.26 Because we still observed that GVT effects were being preserved, the effects of bortezomib may only suppress certain subsets of alloreactive T cells, therefore making it more efficacious in anti-GVHD interventions than some of the commonly used drugs in this field, such as steroid or calcinneurin inhibitors. Tumor relapse is the major cause of death in cGVHD patients.32 Although the occurrence of cGVHD is associated with a decreased incidence of leukemia relapse and improved survival,33 the immunosuppressive therapy used to treat cGVHD can counteract this effect. Although bortezomib has been shown to inhibit alloreactive T cells, which may hinder GVT effects, the direct antitumor effects, as seen in our A20 tumor model, may provide a survival benefit. Also, because bortezomib can protect thymic damage due to cGVHD pathogenesis, the increase in T-cell reconstitution could possibly further enhance the GVT effects.
In conclusion, bortezomib can be a therapeutic option for patients with extensive and steroid refractory cGVHD. Given the encouraging results on GVT in the mouse studies, the results indicate that bortezomib may also allow for the dissociation between cGVHD and GVT.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Monja Metcalf and Weihong Ma for technical help.
This work was supported by NIH National Cancer Institute grant R01CA102282 and NIH National Institute of Allergy and Infectious Diseases grants P01 AI056299, P01 CA142106, and P01 AI 056299. This study was also supported in part by research funding from Millennium (M.A.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or NIH.
Authorship
Contribution: C.-C.S.P., M.A., and W.J.M. authored the manuscript and planned, organized, and performed the research; C.-C.S.P., M.C., A.M., S.K.G., J.T., E.A., K.S., and J.J. performed experiments; and B.R.B., M.A., and W.J.M. supervised the research, reviewed the manuscript, and obtained funding.
Conflict-of-interest disclosure: M.A. received partial research funding from the Millennium Company, and is on the speaker list of the Millennium Company for multiple myeloma. The remaining authors declare no competing financial interests.
Correspondence: William J. Murphy, Department of Dermatology, Internal Medicine, School of Medicine, Comprehensive Cancer Center, University of California, Davis, Sacramento, CA 95817; e-mail: wmjmurphy@ucdavis.edu.
References
Author notes
W.J.M. and M.A. contributed equally to this work.