Key Points
Acute myeloid leukemia (AML) patients present an altered glucose metabolism signature.
A panel of 6 metabolite biomarkers involved in glucose metabolism are identified with prognostic value for cytogenetically normal AML.
Abstract
Acute myeloid leukemia (AML) is a group of hematological malignancies with high heterogeneity. There is an increasing need to improve the risk stratification of AML patients, including those with normal cytogenetics, using molecular biomarkers. Here, we report a metabolomics study that identified a distinct glucose metabolism signature with 400 AML patients and 446 healthy controls. The glucose metabolism signature comprises a panel of 6 serum metabolite markers, which demonstrated prognostic value in cytogenetically normal AML patients. We generated a prognosis risk score (PRS) with 6 metabolite markers for each patient using principal component analysis. A low PRS was able to predict patients with poor survival independently of well-established markers. We further compared the gene expression patterns of AML blast cells between low and high PRS groups, which correlated well to the metabolic pathways involving the 6 metabolite markers, with enhanced glycolysis and trichloracetic acid cycle at gene expression level in low PRS group. In vitro results demonstrated enhanced glycolysis contributed to decreased sensitivity to antileukemic agent arabinofuranosyl cytidine (Ara-C), whereas inhibition of glycolysis suppressed AML cell proliferation and potentiated cytotoxicity of Ara-C. Our study provides strong evidence for the use of serum metabolites and metabolic pathways as novel prognostic markers and potential therapeutic targets for AML.
Introduction
Acute myeloid leukemia (AML) is a group of hematologic neoplasms with diverse genetic abnormalities.1-3 Risk stratification based on cytogenetic characteristics divides AML patients into 3 subgroups, favorable, intermediate, and unfavorable risk cytogenetics,4 with 5-year overall survival (OS) of 55%, 38%, 11%, respectively.5 The cytogenetically normal AML (CN-AML) of the intermediate-risk group, which accounts for about one-half of total AML, is also heterogeneous as shown by inferior prognosis in cases with FLT3-ITD or DNMT3A mutations, and good prognosis in cases with NPM1 mutations or bi-allelic CEBPA mutations in the absence of FLT3-ITD mutations.4,6 In addition, mutations in other genes, such as isocitrate dehydrogenases 1 and 2 (IDH1/2), ASXL1, WT1, and TET2, have been identified in this group recently.7 Because of the heterogenetic nature, many genetic mutations identified so far have yet to demonstrate their ability to confidently predict the outcome for the CN-AML patients.
Previous studies have revealed that the glucose metabolism, including glycolysis and tricarboxylic acid (TCA) cycle, is reprogrammed in many malignancies as shown by the accelerated glycolysis to provide energy/biosynthetic precursors and by the active truncated TCA cycle to produce intermediates for tumor cells.8,9 The altered glucose metabolism is closely associated with the therapeutic resistance and clinical outcome.9-11 High expression and increased activity of lactate dehydrogenase-A in glycolysis induce Taxol resistance in breast cancer cells.12 Overexpression of pyruvate dehydrogenase kinase-3 in colon cancer contributes to hypoxia-induced drug resistance.10 Total lesion glycolysis is associated with the survival of lung adenocarcinoma patients.13 An intermediate metabolite of TCA cycle, 2-hydroxyglutarate (2-HG, consisting of both d- and l-hydroxyglutarate enantiomers), generated by mutated IDH1/2, is considered an “onco-metabolite” and identified to be a prognostic factor of AML in a recent report published by our group.14 Based on these reports, we tested a hypothesis in this study that the glucose metabolism might be modified in AML patients and be associated with the prognosis of the patients.
Mass spectrometry-based metabolomic profiling is sensitive and robust, allowing for simultaneous identification of a large number of metabolites15 and their changes associated with a pathophysiological process. The past few years have witnessed successful applications of this technology in the study of various cancers.16,17 Here, we present a gas chromatography-time-of-flight mass spectrometry (GC-TOFMS)-based metabolomics study of AML serum, which is focused on the glucose metabolism and a panel of serum metabolite markers instead of 2-HG alone, with potential for AML risk stratification.
Methods
Patients and serum samples
A total of 229 de novo AML patients and 260 age- and gender-matched healthy controls (HCs) were enrolled in 2007 to 2010 from the hematology center of Rui Jin Hospital in Shanghai, while another group of participants, including 171 newly diagnosed AML patients and 186 age- and gender-matched HCs, were enrolled in 2011 to 2012 from 6 hematology centers of Hangzhou, Suzhou, Shenyang, Nanjing, Dalian, and Beijing. All participants provided written informed consent in accordance with the regulation of the Institutional Review Boards of the related Universities/Hospitals in agreement with the Declaration of Helsinki. For AML patients, WHO classification, conventional cytogenetic banding assay, and molecular genetic analysis were performed as previously described.1,18 The gene abnormalities were examined in 199 of 233 CN-AML patients. Gene mutations of FLT3-ITD, CEBPA, NPM1, and DNMT3A were analyzed by whole-gene sequencing, and mutational status of MLL-PTD was determined by reverse-transcription polymerase chain reaction (RT-PCR). Cytogenetic groups of patients were classified as favorable, intermediate, and unfavorable risk according to the National Comprehensive Cancer Network guideline.4 The treatment protocols are provided in the supplemental Appendix on the Blood Web site.
Serum samples were collected from patients at diagnosis using the same protocol among all the hematology centers. Overnight fasting peripheral blood samples were collected in the morning and transferred into vacuum blood collection tubes without any anticoagulants. All blood samples were clotted at room temperature for <2 hours and centrifuged at 956 × g for 10 minutes. Serum samples were obtained and stored at −80°C until analysis.
Metabolomic profiling with GC-TOFMS
Metabolomic profiles of all serum samples were achieved using GC-TOFMS platform as previously described.16,17 Samples were randomized prior to GC-TOFMS analysis to decrease experimental drifts. Quality control (QC) samples, which were prepared by mixing equal amounts of serum samples from all enrolled subjects, were used to control intra- and inter-batch variability. QC samples were distributed evenly among the injections for each day. Detailed descriptions of sample preparation and GC-TOFMS analysis methods are provided in the supplemental Appendix.
After the pretreatment of baseline correction, de-noising, smoothing, alignment, time-window splitting, and multivariate curve resolution, raw data containing retention time, intensity, and the mass-to-charge ratio of each peak were obtained. A total of 100 metabolites were identified by the comparison with the internal library built with the standard reference compounds and the National Institute of Standards and Technology library (Wiley registry). The intensity data of these metabolites were used to perform metabolomics profiling analysis. Six metabolites of the glucose metabolism differentially expressed in AML serum, including glycerol-3-phosphate, pyruvate, lactate, citrate, 2-oxoglutarate, and 2-HG, were quantitatively determined from the calibration curves.
Gene expression profiling
Human U133 Plus 2.0 GeneChip (Affymetrix) was used for gene expression profiling. Briefly, the RNA was extracted by using a RNeasy micro kit (Qiagen, GmBH, Germany), labeled with GeneChip 3′ IVT Express Kit (Affymetrix), and hybridized with Human U133 Plus 2.0 GeneChip following the manufacturer’s protocol. The quality of samples and assays was evaluated by measures of the percentage of genes present (mean ± standard deviation: 44.71 ± 1.66) and the ratio of glyceraldehyde-3-phosphate dehydrogenase 3′ to 5′ (mean ± standard deviation: 2.12 ± 0.91).
Quantitative RT-PCR
The expression of metabolic genes involved in glycolysis and TCA cycle was measured by quantitative RT-PCR. 18S rRNA was used as the internal control. The assay was performed using SYBR Premix Ex Taq (Takara, Otsu, Japan) on an Applied Biosystems 7900 Real Time PCR machine (Applied Biosystems, Foster City, CA). All primers are listed in supplemental Table 1.
Cell viability assay
AML cell lines (HL-60, U937, OCI-AML3, THP-1, and KG-1) and primary cells from bone marrow (BM) of de novo AML patients were cultured in RPMI-1640 (Gibco, NY) with 2 mM l-glutamine and 10% fetal bovine serum (Biochrom AG, Berlin, Germany) and maintained at 37°C and 5% CO2. AML cell lines and primary cells were seeded in 96-well plates at a density of 20 000 cells/well and 100 000 cells/well, respectively. For determinations of cytotoxicities of glycolytic inhibitors 2-deoxy-d-glucose (2-DG, Sangon Biotech, Shanghai, China) and dichloroacetate (DCA, Sigma-Aldrich, St. Louis, MO), cells were separately treated in a proper concentration range. 2-DG and antileukemic agent arabinofuranosyl cytidine (Ara-C, Pfizer, New York, NY) were used together to investigate synergistic effect. After treatment of 48 hours, Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) was used to measure cell viability following the manufacturer’s protocol.
Knockdown of glycolytic hexokinase 1 by shRNA
The short hairpin RNA (shRNA) duplex against hexokinase 1 (5′-CAGCCACAGTCAAGATGTT-TTCAAGAGA-AACATCTTGACTGTGGCTG-3′ and 5′-GTCGGTGTCAGTTCTACAA-TTCAAGAGA-TTGTAGAACTGACACCGAC-3′) was synthesized and cloned into pLVX vector (Clontech). The lentivirus was made as follows: shRNA-encoding pLVX vector was cotransfected with pMD2.G and psPAX2 plasmids into HEK293T cell by use of Lipofectamine 2000 (Invitrogen). AML cell lines U937 and OCI-AML3 were infected with the lentivirus in the presence of 8 mg/mL polybrene. After infection for 48 hours, the green fluorescent protein–positive cells were sorted.
Development of prognosis-risk score
As stated in the Introduction, we focused on the glucose metabolism in this study to screen potential metabolite biomarkers for AML risk stratification. Among the total metabolites identified in glucose metabolism, 6 of them were differentially expressed in AML serum. This panel of metabolites was selected for prognostic value assessment. The log2-transformed and then Z-score–normalized quantitative data of these metabolites were used in the analysis.
Clinical outcome was analyzed in CN-AML patients due to the relatively low heterogeneity in this group compared with the other cytogenetic groups. Among 263 patients in this group, only 233 cases with detailed therapeutic information were enrolled for prognostic analysis. A predictive principal component analysis (PCA) model was fitted in the training matrix (n = 134) containing only the above panel of 6 metabolite biomarkers. The PCA model was used to generate prognosis-risk score (PRS) for each patient in training set and to predict PRS for each case in validation set (n = 99). Specifically, the first principal component (a weighted average expression among those 6 metabolites) was applied to obtain PRS for each patient, as it accounted for the largest variability in the data. The median PRS in the training set was used as the cutoff value to divide the 2 sets of patients into 2 groups: those with low and high PRSs.
Data treatment and statistical analysis
The metabolomic data were normalized using internal standard L-2-chlorophenylalanine, and intra-batch and inter-batch calibration was achieved using QC samples (see supplemental Methods for details). Multivariate statistical model of orthogonal partial least square discriminate analysis (OPLS-DA) was constructed with the software SIMCA-P+ (version 11.0, Umetric, Umea, Sweden). Wilcoxon rank-sum test with Bonferroni correction was performed to identify differentially expressed metabolites. Altered metabolic pathways in AML were analyzed by means of the quantitative enrichment analysis algorithm represented in metabolite set enrichment analysis method.19 Visualization of metabolic pathways was achieved by using metscape 2 running on cytoscape.20,21
The clinical characteristics, molecular features, and outcomes were compared between CN-AML with low and high PRSs. For prognosis analysis, complete remission (CR) was defined as previously described.22 OS was measured as time from disease diagnosis to death from any cause or censoring for patients alive at the time of their final follow-up. Event-free survival (EFS) was defined as time from disease diagnosis until removal from study because of failure to achieve CR, relapse, or death from any cause. OS and EFS were compared between low and high PRS groups by means of Kaplan-Meier method followed with log-rank test. Multivariate Cox regression analysis was applied to evaluate the prognostic value of PRS after adjustment for other confounding factors. The proportional-hazards assumption was checked for each variable before fitting Cox models.
Gene expression data were normalized with MAS 5.0 method.23 Subsequently, log2 transformation was executed. T test with Bonferroni correction was carried out to find differentially expressed probes between the low and high PRS groups.
Calculation of IC50 values and analysis of the synergistic effect were executed in CompuSyn software (ComboSyn, Inc., Paramus, NJ).
Statistical analyses were performed with use of R (version 2.15.0, www.r-project.org) and SAS software, version 9.3 (www.sas.com). All statistical tests were 2-sided, and P values < .05 were considered statistically significant.
Results
Metabolic alteration and distinct glucose metabolism of AML
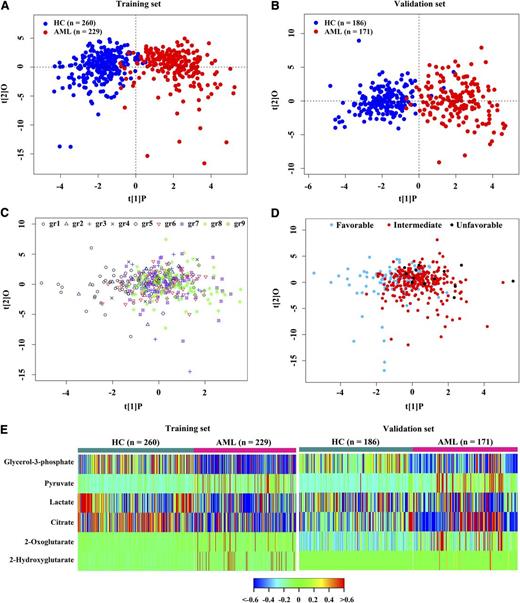
We used the samples from the hematology center of Rui Jin Hospital as the training set and the samples from 6 hematology centers as the validation set. There were no significant differences in age, gender, serum alanine aminotransferase, or creatinine between the 2 sample sets or between HC and AML patients in each set (Table 1). A total of 100 metabolites was identified in this study (supplemental Table 2). The metabolomic profile of the AML group represented by these 100 metabolites was distinct from that of the HC (Figure 1A-B), as evidenced by robust OPLS-DA models established in the training set (R2Y = 0.77, Q2 = 0.69) and validation set (R2Y = 0.77, Q2 = 0.66). Among AML patients, no significant differences in serum metabolite levels were observed among distinct WHO subtypes or cytogenetic risk groups, as shown by the poor OPLS-DA models with low fitting parameters (R2Y = 0.26 and Q2 = 0.04 for WHO subtypes, and R2Y = 0.21 and Q2 = −0.06 for cytogenetic risk groups) (Figure 1C-D).
Basic characteristics of 446 HC and 400 AML patients in training and validation sets
| Variable . | Training set . | Validation set . | P value for difference between training and validation sets* . | ||||
|---|---|---|---|---|---|---|---|
| HC (n = 260) . | AML (n = 229) . | P value* . | HC (n = 186) . | AML (n = 171) . | P value* . | ||
| Age, years | .11 | .45 | .19 | ||||
| Median | 44.00 | 47.00 | 45.00 | 47.00 | |||
| Range | 17-82 | 15-82 | 24-76 | 15-89 | |||
| Gender, no. (%) | .90 | .16 | .94 | ||||
| Male | 153 (58.85) | 136 (59.39) | 116 (62.37) | 94 (54.97) | |||
| Female | 107 (41.15) | 93 (40.61) | 70 (37.63) | 77 (45.03) | |||
| ALT, U/L | .63 | .06 | .75 | ||||
| Median | 21.00 | 22.00 | 22.00 | 19.50 | |||
| Range | 10.00-166.00 | 5.00-459.00 | 9.00-76.00 | 3.00-288.00 | |||
| AST, U/L | .01 | .23 | .49 | ||||
| Median | 21.00 | 24.00 | 22.00 | 21.00 | |||
| Range | 13.00-55.00 | 3.00-607.00 | 12.00-43.00 | 3.00-348.00 | |||
| Serum creatinine groups, no.† | — | .30 | .88 | ||||
| ≤230 μmol/L | 260 | 229 | 186 | 170 | |||
| >230 μmol/L | 0 | 0 | 0 | 1 | |||
| Hepatic or renal function, no.‡ | .07 | .07 | .59 | ||||
| Normal | 259 | 224 | 186 | 168 | |||
| Abnormal | 1 | 5 | 0 | 3 | |||
| WBC, 109/L | .55 | ||||||
| Median | 8.00 | 7.30 | |||||
| Range | 0.10-292.20 | 0.08-290.00 | |||||
| Platelet, 109/L | .72 | ||||||
| Median | 39.00 | 37.00 | |||||
| Range | 4.00-500.00 | 2.00-899.00 | |||||
| BM blasts, % | .53 | ||||||
| Median | 66.00 | 65.00 | |||||
| Range | 20.00-99.00 | 18.00-97.00 | |||||
| Cytogenetics, no. (%) | .24 | ||||||
| t(15;17)/PML-RARA | 38 (16.59) | 19 (11.11) | |||||
| t(8;21)/AML1-ETO | 28 (12.23) | 19 (11.11) | |||||
| inv(16;16)/CBFβ-MYH11 | 7 (3.06) | 12 (7.02) | |||||
| CN-AML§ | 148 (64.63) | 115 (67.25) | |||||
| Unfavorable|| | 8 (3.49) | 6 (3.51) | |||||
| Variable . | Training set . | Validation set . | P value for difference between training and validation sets* . | ||||
|---|---|---|---|---|---|---|---|
| HC (n = 260) . | AML (n = 229) . | P value* . | HC (n = 186) . | AML (n = 171) . | P value* . | ||
| Age, years | .11 | .45 | .19 | ||||
| Median | 44.00 | 47.00 | 45.00 | 47.00 | |||
| Range | 17-82 | 15-82 | 24-76 | 15-89 | |||
| Gender, no. (%) | .90 | .16 | .94 | ||||
| Male | 153 (58.85) | 136 (59.39) | 116 (62.37) | 94 (54.97) | |||
| Female | 107 (41.15) | 93 (40.61) | 70 (37.63) | 77 (45.03) | |||
| ALT, U/L | .63 | .06 | .75 | ||||
| Median | 21.00 | 22.00 | 22.00 | 19.50 | |||
| Range | 10.00-166.00 | 5.00-459.00 | 9.00-76.00 | 3.00-288.00 | |||
| AST, U/L | .01 | .23 | .49 | ||||
| Median | 21.00 | 24.00 | 22.00 | 21.00 | |||
| Range | 13.00-55.00 | 3.00-607.00 | 12.00-43.00 | 3.00-348.00 | |||
| Serum creatinine groups, no.† | — | .30 | .88 | ||||
| ≤230 μmol/L | 260 | 229 | 186 | 170 | |||
| >230 μmol/L | 0 | 0 | 0 | 1 | |||
| Hepatic or renal function, no.‡ | .07 | .07 | .59 | ||||
| Normal | 259 | 224 | 186 | 168 | |||
| Abnormal | 1 | 5 | 0 | 3 | |||
| WBC, 109/L | .55 | ||||||
| Median | 8.00 | 7.30 | |||||
| Range | 0.10-292.20 | 0.08-290.00 | |||||
| Platelet, 109/L | .72 | ||||||
| Median | 39.00 | 37.00 | |||||
| Range | 4.00-500.00 | 2.00-899.00 | |||||
| BM blasts, % | .53 | ||||||
| Median | 66.00 | 65.00 | |||||
| Range | 20.00-99.00 | 18.00-97.00 | |||||
| Cytogenetics, no. (%) | .24 | ||||||
| t(15;17)/PML-RARA | 38 (16.59) | 19 (11.11) | |||||
| t(8;21)/AML1-ETO | 28 (12.23) | 19 (11.11) | |||||
| inv(16;16)/CBFβ-MYH11 | 7 (3.06) | 12 (7.02) | |||||
| CN-AML§ | 148 (64.63) | 115 (67.25) | |||||
| Unfavorable|| | 8 (3.49) | 6 (3.51) | |||||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; WBC, white blood cell.
P values were calculated by means of nonparametric Wilcoxon rank-sum test for continuous variables and χ-square test for categorical variables. — indicates not applicable.
230 μmol/L is equal to 2× normal value.
Hepatic abnormality as defined by ALT >2.5×normal value or AST >2.5× normal value, while renal abnormality as defined by creatinine >2.5× normal value.
CN-AML: cases having no cytogenetically identifiable abnormalities.
Unfavorable: inv(3)/t(3;3), t(9;22), 11q23 abnormalities, −5, −7, del(5q),del(7p), and complex karyotype.
Metabolic alteration of AML. OPLS-DA score plots showed a global metabolic difference between AML and HC in the training set (A) and validation set (B). (C) Metabolomic profiles of 9 AML subtypes based on WHO classification. (D) Metabolomic profiles of different cytogenetic risk groups of AML patients. (E) Heat map showed 6 differentially expressed serum metabolites involved in glucose metabolism in AML compared with HC. gr1, AML with t(15;17); gr2, AML with t(8;21); gr3, AML with t(16;16); gr4, AML with minimal differentiation; gr5, AML without maturation; gr6, AML with maturation; gr7, acute myelomonocytic leukemia; gr8, acute monoblastic/monocytic leukemia; g9, acute erythroid leukemia.
Metabolic alteration of AML. OPLS-DA score plots showed a global metabolic difference between AML and HC in the training set (A) and validation set (B). (C) Metabolomic profiles of 9 AML subtypes based on WHO classification. (D) Metabolomic profiles of different cytogenetic risk groups of AML patients. (E) Heat map showed 6 differentially expressed serum metabolites involved in glucose metabolism in AML compared with HC. gr1, AML with t(15;17); gr2, AML with t(8;21); gr3, AML with t(16;16); gr4, AML with minimal differentiation; gr5, AML without maturation; gr6, AML with maturation; gr7, acute myelomonocytic leukemia; gr8, acute monoblastic/monocytic leukemia; g9, acute erythroid leukemia.
A total of 10 metabolites involved in the glucose metabolism was identified in this study, including glucose, glycerol-3-phosphate, pyruvate, and lactate in glycolysis, and citrate, 2-oxoglutarate, succinate, fumarate, malate, and 2-HG in the TCA cycle. Six of them were differentially expressed in AML serum in both training and validation sets (Bonferroni-corrected P < .05, false discovery rate q < 0.05) (Figure 1E). Among these 6 metabolites, glycerol-3-phosphate, lactate, and citrate were decreased, whereas pyruvate, 2-oxoglutarate, and 2-HG were increased in AML serum compared with HC (Figure 1E; supplemental Table 3).
In the remaining 90 metabolites, 41 of them were significantly modified in AML serum in both training and validation sets (Bonferroni-corrected P < .05, false discovery rate q < 0.05) (supplemental Figure 1A). The total of 47 modified metabolites included alcohols, amino acids, carbohydrates, fatty acids, nucleosides, organic acids, and others (supplemental Figure 1A). A total of 45 metabolic pathways was found dysregulated in AML based on the analysis of the quantitative enrichment analysis algorithm of the metabolite set enrichment analysis method (Bonferroni-corrected P < .05) (supplemental Figures 1B and 2-5).19
Development of the PRS of metabolite biomarkers and its association with clinical outcomes in CN-AML
A distinct glucose metabolism signature was identified in AML as demonstrated by the significant modification of 6 of 10 identified serum metabolites in this pathway. As mentioned above, we hypothesized glucose metabolism was associated with the prognosis of AML patients. Therefore, we chose this panel of 6 metabolites in glucose metabolism to assess prognostic potential (Table 2).
A panel of 6 metabolites with prognostic value for CN-AML
| Metabolite‡ . | Importance score for OS and EFS* . | Regression weight† . | Median, range, µg/mL . |
|---|---|---|---|
| Lactate | 74.17 | −0.67 | 3 393.09 (444.08-15 073.18) |
| 2-Oxoglutarate | 57.12 | −0.51 | 1.34 (1.20-1.44) |
| Pyruvate | 53.89 | −0.48 | 0.53 (0-54.84) |
| 2-HG | 12.3 | −0.11 | 3.63 (3.54-530.60) |
| Glycerol-3-phosphate | 8.05 | −0.07 | 0.76 (0.66-1.51) |
| Citrate | −23.54 | 0.21 | 2.57 (0.80-6.23) |
| Metabolite‡ . | Importance score for OS and EFS* . | Regression weight† . | Median, range, µg/mL . |
|---|---|---|---|
| Lactate | 74.17 | −0.67 | 3 393.09 (444.08-15 073.18) |
| 2-Oxoglutarate | 57.12 | −0.51 | 1.34 (1.20-1.44) |
| Pyruvate | 53.89 | −0.48 | 0.53 (0-54.84) |
| 2-HG | 12.3 | −0.11 | 3.63 (3.54-530.60) |
| Glycerol-3-phosphate | 8.05 | −0.07 | 0.76 (0.66-1.51) |
| Citrate | −23.54 | 0.21 | 2.57 (0.80-6.23) |
PRS of each sample is calculated according to followed equation: PRS = (−0.67) × lactate + (−0.51) × 2-oxoglutarate + (−0.48) × pyruvate + (−0.11) × 2-HG + (−0.07) × glycerol-3-phosphate + 0.21 × citrate.
Importance score was computed by means of the SuperPC algorithm. Negative score means that increasing in value of metabolite indicates better survival, whereas positive score means that increasing in value of metabolite indicates worse survival.
The weight of the first principal component in PCA model.
Metabolites are confirmed by standards.
The prognostic value of the panel of those metabolites was first evaluated in CN-AML patients. The estimated 2-year OS and EFS rates for these 233 patients were 38.20% (95% CI: 31.60% to 46.20%) and 31.70% (95% CI: 25.50% to 39.40%), respectively. There were no significant differences between training (n = 134) and validation (n = 99) sets for clinical characteristics, gene mutations, CR rate, median OS, and median EFS (supplemental Table 4).
A predictive PCA model was fitted in the training set, including the above 6 metabolite markers (Table 2). Meanwhile, each of the metabolites was evaluated in association with the survival of patients using the importance scores computed by SuperPC algorithm.24 Lactate, 2-oxoglutarate, pyruvate, 2-HG, and glycerol-3-phosphate were found to be negatively associated with the OS and EFS of patients, whereas citrate was positively associated (Table 2). As described in the Methods section, patients in 2 sets were divided into low and high PRS groups based on the PRS values from PCA model. There were no significant differences in clinical characteristics and molecular features between these 2 groups (supplemental Table 4).
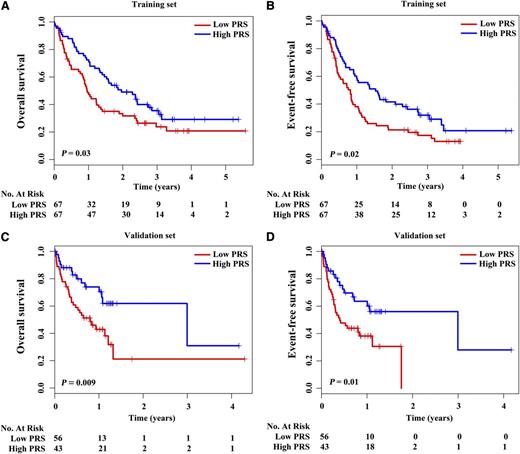
In the training set, patients with low PRS achieved a shorter median OS and median EFS (352 days and 299 days, respectively) than those with high PRS (716 days and 559 days, respectively) (P = .03 for OS and P = .02 for EFS) (Figure 2; supplemental Table 4). The estimated 2-year OS rates were 31.70% vs 49.10%, and the estimated 2-year EFS rates were 21.30% vs 41.50% between these 2 groups (supplemental Table 4). A similar pattern was observed in the validation set, those with low PRS showing worse median OS and EFS (293 days and 146 days, respectively) than those with high PRS (1093 days and 1093 days, respectively) (P = .009 for OS and P = .01 for EFS) (Figure 2; supplemental Table 4). The estimated 2-year OS rates were 21.20% vs 61.90%, and the estimated 2-year EFS rates were 0% vs 56.00% for the 2 groups (supplemental Table 4).
Prognostic analysis of CN-AML with low and high PRSs. Survival curves showed OS and EFS of CN-AML with low and high PRSs in the training set (A-B) and the validation set (C-D). P values were calculated by means of log-rank test.
Prognostic analysis of CN-AML with low and high PRSs. Survival curves showed OS and EFS of CN-AML with low and high PRSs in the training set (A-B) and the validation set (C-D). P values were calculated by means of log-rank test.
Univariate analysis demonstrated that low PRS, age, WBC count, percentage of BM blasts, FLT3-ITD mutations, DNMT3A mutations, and high 2-HG were poor risk factors, whereas CEBPA biallelic mutations was a favorable factor for OS and EFS (supplemental Table 5). To investigate whether low PRS could predict poor survival independently of well-known prognostic factors, multivariate Cox regression analysis was performed in combined training and validation sets. Low PRS predicted inferior OS (HR = 1.80, P = .008) and poor EFS (HR = 1.75, P = .008) after adjustment for well-known prognostic parameters, including age, WBC count, percentage of BM blasts, treatment protocols, FLT3-ITD mutations, CEBPA biallelic mutations, NPM1 mutations, DNMT3A mutations, MLL-PTD, and 2-HG (Table 3). To better evaluate the prognostic value of gene abnormalities, we combined FLT3-ITD, DNMT3A, and MLL-PTD as an unfavorable gene panel, and CEBPA and NPM1 as a favorable gene panel. Low PRS was still a predictor for poor OS and EFS in multivariate model (supplemental Table 6). In addition, when senile patients who received less intensive chemotherapy were excluded, low PRS still predicted inferior OS and EFS (supplemental Table 7).
Multivariate analysis of PRS of metabolite biomarkers as a prognostic factor for OS and EFS in combined training and validation sets
| Variable . | OS . | EFS . | ||
|---|---|---|---|---|
| HR (95% CI)* . | P value . | HR (95% CI)* . | P value . | |
| PRS† | 1.80 (1.17-2.77) | .008 | 1.75 (1.15-2.64) | .008 |
| Age‡ | 1.02 (1.00-1.04) | .06 | 1.02 (1.00-1.03) | .13 |
| WBC‡ | 1.007 (1.003-1.010) | .001 | 1.006 (1.002-1.010) | .002 |
| BM blasts, %‡ | 1.008 (0.999-1.017) | .10 | 1.009 (1.000-1.018) | .05 |
| Treatment protocols§ | .25 | .10 | ||
| T2 vs T1 | 0.62 (0.34-1.11) | .11 | 0.54 (0.31-0.96) | .04 |
| T3 vs T1 | 1.03 (0.56-1.89) | .93 | 0.77 (0.42-1.41) | .39 |
| FLT3-ITD|| | 0.78 (0.43-1.44) | .44 | 0.86 (0.48-1.54) | .62 |
| CEBPA¶ | 0.36 (0.17-0.76) | .008 | 0.49 (0.26-0.93) | .03 |
| NPM1|| | 0.87 (0.54-1.38) | .55 | 0.81 (0.52-1.27) | .36 |
| DNMT3A|| | 1.70 (0.94-3.06) | .08 | 1.56 (0.88-2.75) | .13 |
| MLL-PTD# | 0.93 (0.41-2.08) | .86 | 1.42 (0.70-2.87) | .33 |
| 2-HG** | 1.60 (1.00-2.56) | .05 | 1.83 (1.16-2.88) | .009 |
| Variable . | OS . | EFS . | ||
|---|---|---|---|---|
| HR (95% CI)* . | P value . | HR (95% CI)* . | P value . | |
| PRS† | 1.80 (1.17-2.77) | .008 | 1.75 (1.15-2.64) | .008 |
| Age‡ | 1.02 (1.00-1.04) | .06 | 1.02 (1.00-1.03) | .13 |
| WBC‡ | 1.007 (1.003-1.010) | .001 | 1.006 (1.002-1.010) | .002 |
| BM blasts, %‡ | 1.008 (0.999-1.017) | .10 | 1.009 (1.000-1.018) | .05 |
| Treatment protocols§ | .25 | .10 | ||
| T2 vs T1 | 0.62 (0.34-1.11) | .11 | 0.54 (0.31-0.96) | .04 |
| T3 vs T1 | 1.03 (0.56-1.89) | .93 | 0.77 (0.42-1.41) | .39 |
| FLT3-ITD|| | 0.78 (0.43-1.44) | .44 | 0.86 (0.48-1.54) | .62 |
| CEBPA¶ | 0.36 (0.17-0.76) | .008 | 0.49 (0.26-0.93) | .03 |
| NPM1|| | 0.87 (0.54-1.38) | .55 | 0.81 (0.52-1.27) | .36 |
| DNMT3A|| | 1.70 (0.94-3.06) | .08 | 1.56 (0.88-2.75) | .13 |
| MLL-PTD# | 0.93 (0.41-2.08) | .86 | 1.42 (0.70-2.87) | .33 |
| 2-HG** | 1.60 (1.00-2.56) | .05 | 1.83 (1.16-2.88) | .009 |
Hazard ratios (HRs) >1 correspond to an increased risk of death/relapse compared with the lower values of continuous variables or the reference group of categorical variables.
Low vs high.
Age, WBC, and percentage of BM blasts as continuous variables.
T1, DA regimen; T2, homoharringtonine-based treatment; T3, individualized treatment of elderly patients.
Mutant vs wild-type.
Biallelic CEBPA mutants vs monoallelic CEBPA mutants/wild type.
Positive vs negative.
High vs low as we previously reported.14
Verification of the modified glucose metabolism of CN-AML with low PRS by gene-expression profiling
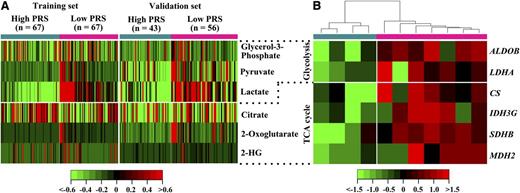
The regression weights of metabolite biomarkers in the predictive PCA model (Table 2) indicated that lactate, 2-oxoglutarate, pyruvate, 2-HG, and glycerol-3-phosphate were negatively correlated to PRS value, whereas citrate was positively correlated. Correspondingly, lactate, 2-oxoglutarate, pyruvate, 2-HG, and glycerol-3-phosphate were increased in the low PRS group, whereas citrate was reduced (Figure 3A). These analyses suggested glycolysis and a truncated TCA cycle were both enhanced in the low PRS group. Accordingly, gene expression profiling in BM blasts from 7 patients with low PRS and 4 patients with high PRS verified the modified glucose metabolism of patients with low PRS, as demonstrated by the upregulated glycolytic genes ALDOB/LDHA and TCA cycle genes CS/IDHG/SDHB/MDH2 in this group (P < .05) (Figure 3B).
Glucose metabolism and mRNA expression of related metabolic genes in CN-AML patients with low and high PRSs. (A) Heat map showed 6 serum metabolite biomarkers between low and high PRS groups. (B) Heat map showed changes in the expression of genes involved in glucose metabolism of low and high PRS groups. Shades of red and green represented high or low expression (see color scale). Each column represented a patient with low PRS (denoted by deep pink bar) or a patient with high PRS (denoted by deep sky blue bar).
Glucose metabolism and mRNA expression of related metabolic genes in CN-AML patients with low and high PRSs. (A) Heat map showed 6 serum metabolite biomarkers between low and high PRS groups. (B) Heat map showed changes in the expression of genes involved in glucose metabolism of low and high PRS groups. Shades of red and green represented high or low expression (see color scale). Each column represented a patient with low PRS (denoted by deep pink bar) or a patient with high PRS (denoted by deep sky blue bar).
Aberrant glycolysis pathway as potential therapeutic target for AML
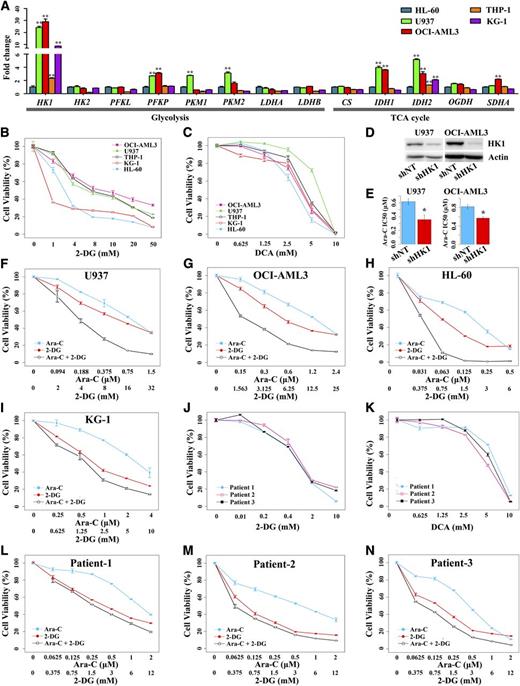
To uncover the biological role of the modified glucose metabolism in patients with low PRS, 5 AML cell lines, HL-60, U937, OCI-AML3, THP-1, and KG-1 were chosen for investigation of their metabolic features. Compared with HL-60, the expression of glycolytic genes and TCA cycle genes was increased in the other 4 cell lines (Figure 4A), indicating glycolysis and TCA cycle were both enhanced in these 4 cell lines.
The mRNA expression of genes involved in glucose metabolism and the biological role of aberrant glycolysis in AML cells. (A) Assay of the expression of genes involved in glucose metabolism in 5 AML cell lines, HL-60, U937, OCI-AML3, THP-1, and KG-1. Target gene expression in U937, OCI-AML3, THP-1, and KG-1 was normalized to those in HL-60 (which were set at 1) and presented as fold changes relative to HL-60. **P < .01 vs HL-60. (B-C) Cell viability curves of AML cell lines treated with glycolytic inhibitors 2-DG and DCA, respectively. (D) Western blot showed downregulation of HK1 in U937 and OCI-AML3 by shRNA. (E) Increased sensitivity to Ara-C in U937 and OCI-AML3 with reduced HK1 expression. *P < .05 vs shNT. (F-I) Synergistic effect of 2-DG and Ara-C on U937, OCI-AML3, HL-60, and KG-1, respectively. (J-K) Cell viability curves of AML primary blast cells treated with glycolytic inhibitors 2-DG and DCA, respectively. (L-N) Synergistic effect of 2-DG and Ara-C on AML primary blast cells of patient 1, patient 2, and patient 3, respectively. Each measure was performed with at least 3 duplicates and was expressed as mean ± SE.
The mRNA expression of genes involved in glucose metabolism and the biological role of aberrant glycolysis in AML cells. (A) Assay of the expression of genes involved in glucose metabolism in 5 AML cell lines, HL-60, U937, OCI-AML3, THP-1, and KG-1. Target gene expression in U937, OCI-AML3, THP-1, and KG-1 was normalized to those in HL-60 (which were set at 1) and presented as fold changes relative to HL-60. **P < .01 vs HL-60. (B-C) Cell viability curves of AML cell lines treated with glycolytic inhibitors 2-DG and DCA, respectively. (D) Western blot showed downregulation of HK1 in U937 and OCI-AML3 by shRNA. (E) Increased sensitivity to Ara-C in U937 and OCI-AML3 with reduced HK1 expression. *P < .05 vs shNT. (F-I) Synergistic effect of 2-DG and Ara-C on U937, OCI-AML3, HL-60, and KG-1, respectively. (J-K) Cell viability curves of AML primary blast cells treated with glycolytic inhibitors 2-DG and DCA, respectively. (L-N) Synergistic effect of 2-DG and Ara-C on AML primary blast cells of patient 1, patient 2, and patient 3, respectively. Each measure was performed with at least 3 duplicates and was expressed as mean ± SE.
In view of the fact that the HK1, a gene key to glycolysis pathway, showed significant variation in above the 5 cell lines (P = 1.25 × 10−8) (Figure 4A), we tried 2 glycolytic inhibitors, 2-DG and DCA, to treat these cells. The proliferations of all 5 cell lines were inhibited in a dose-dependent manner under the separate treatment of 2-DG and DCA (Figure 4B-C). Next, we evaluated the effect of glycolysis on drug sensitivity. We first explored the association between glycolysis and the sensitivity to Ara-C, a common drug used in AML chemotherapy. Enhanced glycolysis was associated with decreased sensitivity as demonstrated by the higher IC50 (median inhibition concentration) values of Ara-C for U937, OCI-AML3, THP-1, and KG-1 cells harboring increased glycolysis compared with HL-60 harboring a relatively low level of glycolysis (P < .05) (supplemental Figure 6A). We downregulated the expression of HK1 in U937 and OCI-AML3 using the shRNA technology and observed significantly increased Ara-C sensitivity for these 2 cell lines (Figure 4D-E). The results demonstrated that enhanced glycolysis in leukemic cells contributed to decreased Ara-C sensitivity. Thus, we used 2-DG and Ara-C together to treat AML cell lines. A synergistic effect was observed between these 2 drugs for all 5 AML cell lines (Figure 4F-I; supplemental Table 8), demonstrating that inhibiting glycolysis potentiated the cytotoxicity induced by Ara-C in AML cells.
It was notable that AML primary blast cells from de novo patients presenting distinct glycolysis were all sensitive to glycolytic inhibition as shown by the marked dose-dependent inhibition on the proliferation of these cells by 2-DG or DCA treatment (supplemental Figure 6B and Figure 4J-K). The use of 2-DG also potentiated cytotoxicity of Ara-C in AML primary cells (Figure 4L-N; supplemental Table 8). In addition, 2-DG showed reduced cytotoxicity in healthy monocytes, as demonstrated by the higher IC50 value for healthy monocytes compared with the AML primary cells (P = .004) (supplemental Figure 6C). No synergism was observed between 2-DG and Ara-C for healthy monocytes (supplemental Table 8).
Discussion
AML has been studied thoroughly at the facets of epigenomic and genomic sequencing, gene transcription, and protein expression patterns.6,25,26 However, a comprehensive metabolic signature for this group of diseases is still lacking. Hence, we carried out a serum metabolomic study for newly diagnosed AML patients and showed that 47 metabolites were differentially expressed and 45 metabolic pathways were altered. Enhanced glycolysis was observed in AML as previously reported in many tumors. In cancer cells, the mitochondrion converts its original role as simply a “power house” to a new role as a biosynthetic hub, where more intermediates and anabolic precursors are produced through carboxylation of pyruvate and glutaminolysis, to support cell proliferation, an activity known as anaplerosis.27,28 Increased serum levels of pyruvate and 2-oxoglutarate in AML patients suggested an anaplerotic activity in leukemic cells (supplemental Figures 1A and 2). d-Ribose phosphate is an intermediate in the pentose phosphate pathway and a precursor of de novo purine synthesis. Reduced d-ribose phosphate in AML serum suggested an increased purine synthesis (supplemental Figures 1A and 2C).
Metabolic pathway analysis suggested that glycine/serine/threonine metabolism (supplemental Figure 3A) and methionine/cysteine metabolism (supplemental Figure 3B) were accelerated to increase the pyruvate production, which is in need for mitochondrial respiration and proliferation.29 Free fatty acids, the intermediates in synthesis of unsaturated fatty acids, were all downregulated in AML serum (supplemental Figures 1A and 4), indicating increased consumption of fatty acids for lipid synthesis by leukemic cells. Because of the scarcity of BM samples from AML patients, we were unable, in this work, to conduct a metabolomic analysis for leukemic blasts.
A panel of 6 serum metabolites involved in glucose metabolism has independent prognostic value in CN-AML patients. Low PRS from the PCA model fitted by these 6 metabolites predicted poor OS and EFS independent of other prognostic markers. In addition, our data suggested that metabolic signature was a unique feature of AML independent of cytogenetics risk, because there was no significant metabolic difference among distinct cytogenetics risk groups.
As mentioned above, low PRS was linked to enhanced glycolysis and TCA cycle. Compared with HL-60 cells, U937, OCI-AML3, THP-1, and KG-1 cells showed significantly increased glycolysis and TCA cycle. Enhanced glycolysis in these leukemic cells contributed to reduced sensitivity to Ara-C. In the present work, we found that inhibition of the glycolysis activity suppressed the proliferation of AML cell lines and primary AML cells. Moreover, glycolytic inhibitor 2-DG synergized with Ara-C to enhance its cytotoxic effects on both cell lines as well as primary blast cells. Taken together, our results show that a panel of serum metabolite markers, indicative of glucose metabolism activity, can predict the AML patients with relatively good or poor prognosis. The study also suggests that glucose metabolism, especially the glycolysis pathway, may be a potential target for AML therapy and that a combination of glycolytic inhibitor and chemotherapeutic agent may be a novel strategy for AML treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Shu-Min Xiong and Bin Chen (Rui Jin Hospital, SJTU School of Medicine) for hematologic morphological and cytogenetic analysis. Wei-Na Shen/Zhao Liu/Wei-Na Zhang/Yang Li/Song-Fang Wu (Rui Jin Hospital, SJTU School of Medicine), Yun-Gui Wang/Qiu-Ling Ma (First Hospital, Zhejiang University School of Medicine), Hong Liu (First Hospital, Soochow University School of Medicine), Zhi-Jie Kang (Second Hospital, Dalian Medical University), and Hong-Hu Zhu (Institute of Hematology of Peking University and People’s Hospital) are appreciated for collection and storage of AML samples. Yi Chi/Xin Qi/Lin-Jing Zhao/Yun-Ping Qiu /Xiao-Yan Wang/Jing-Lei Yang/Fen Li (Shanghai Jiao Tong University) and Wen Liao (Shanghai University of Traditional Chinese Medicine) are given thanks for serum preparation of GC-TOFMS. The authors also are grateful to Li-Juan Zhao (Boston Children's Hospital and Harvard Medical School) for critically reviewing the manuscript.
This work was supported by China 973 Program (2013CB966800, 2010CB529200), Special Grant of MOH (201202003), Mega-projects of Scientific Research for the 12th Five-Year Plan (2013ZX09303302), National 863 Program (2012AA02A505), the Samuel Waxman Cancer Research Foundation Co-Principal Investigator Program, and the Shanghai Municipal Natural Science Foundation (12ZR1418400).
Authorship
Contribution: Z.C., S.-J.C., and W.J. were the principal investigators who conceived the study; W.-L.C., J.-H.W., A.-H.Z., Y.-H.W., and X.X. designed and performed the research; W.-L.C. and W.J. wrote the manuscript; W.-L.C., J.-H.W., Y.-H.W., Z.C., S.J.C., and W.J. revised the manuscript; all authors agreed on the final version; W.-L.C., J.-H.W., Y.-H.W., J.-M.L., J.-Q.M., Y.-M.Z., Y.-F.L., Y.-Y.W., J.J., H.H., D.-P.W., Y.L., X.-J.Y., J.-S.Y., J.-Y.L., S.W., and X.-J.H. enrolled patients to this study and analyzed the clinical and molecular data; W.-L.C., J.-H.W., A.-H.Z., and Y.-H.W. carried out the metabolomic experiment; and W.-L.C., J.-H.W., T.-L.C., and B.-S.W. contributed to statistical analysis of the data in this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sai-Juan Chen, State Key Laboratory of Medical Genomics, Shanghai Institute of Hematology, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, 197 Rui Jin Rd II, Shanghai, 200025, China; e-mail: sjchen@stn.sh.cn; and Wei Jia, University of Hawaii Cancer Center, Honolulu, HI 96813; e-mail: wjia@cc.hawaii.edu.
References
Author notes
W.-L.C., J.-H.W., A.-H.Z., X.X., and Y.-H.W. contributed equally to this study.