Key Points
Ncor2 is essential for HSC emergence in zebrafish.
Ncor2 inhibits Fos-Vegfd signaling through recruitment of histone deacetylase 3 (Hdac3).
Abstract
Nuclear receptor corepressors (Ncors) are important for developmental and homeostatic processes in vertebrates, which exert transcriptional repression by coordinating with histone deacetylases. However, little is known about their roles in definitive hematopoiesis. In this study, we show that in zebrafish, ncor2 is required for hematopoietic stem cell (HSC) development by repressing fos-vegfd signaling. ncor2 is specifically expressed in the aorta-gonad-mesonephros (AGM) region in zebrafish embryos. ncor2 deficiency reduced the population of HSCs in both the AGM region and T cells in the thymus. Mechanistically, ncor2 knockdown upregulated fos transcription by modulating the acetylation level in the fos promoter region, which then enhanced Vegfd signaling. Consequently, the augmented Vegfd signaling induced Notch signaling to promote the arterial endothelial fate, therefore, possibly repressing the hemogenic endothelial specification, which is a prerequisite for HSC emergence. Thus, our findings identify a novel regulatory mechanism for Ncor2 through Fos-Vegfd-Notch signaling cascade during HSC development in zebrafish embryos.
Introduction
Hematopoietic stem cells (HSCs) are capable of self-renewing and differentiating into all lineages of blood cells, thus maintaining the homeostasis of the blood system. The lack of sufficient donors to provide a source of HSCs for clinical use makes the production of HSCs urgent, in vitro or ex vivo. The iPS technology holds great promise for such purpose in regenerative medicine. For example, several studies have reported successful induction of blood cells in vitro.1-4 However, the generation of transplantable and functional HSCs in a dish has so far been unsuccessful, which is most likely attributed to the largely unknown molecular mechanism underlying HSC development during embryogenesis and adulthood in vivo.
In vertebrate embryos, HSCs directly emerge from the ventral wall of the dorsal aorta (ie, hemogenic endothelium) through a process known as endothelial-to-hematopoietic transition (EHT).5-7 Many signaling pathways and genes involved in artery establishment and HSC development and function have been well studied, however, our understanding on the specification of hemogenic endothelium has only just begun. For example, only a few of factors involved in this process have thus far been reported, such as F2r,8 Gfi1,9 Runx1,10 and Scl.11
Nuclear receptor corepressors (Ncors) are large proteins, which coordinate with histone deacetylases (Hdacs) to exert transcriptional repression.12,13 Knocking out Ncor1 in mice leads to impaired definitive erythropoiesis and arrested T-cell development.14 Ncor2 is necessary for zebrafish primitive hematopoiesis but the underlying mechanisms remain elusive.15 Usually, Ncors repress transcriptional activities of target genes through recruitment of Hdacs. As a partner of Ncor2, Hdac1 is critical for HSC emergence in zebrafish16 and Hdac3 is known to be necessary for HSC proliferation.17,18 Considering that Ncor2 and Hdacs form a multiunit complex to exert transcriptional repression and that both are involved in hematopoiesis, it is very possible that the interaction of Ncor2 with Hdacs might be required for HSC development.
c-Fos (encoded by fos) has been reported to be essential for the activities of HSCs in mice4,19,20 and inhibition of Hdacs can lead to upregulation of fos gene expression in the nervous system.21,22 However, whether this induction is conserved during HSC development remains unclear. Vegfd, also called c-Fos induced growth factor, usually binds to VEGFR3 or VEGFR2 to regulate lymphangiogenesis and/or angiogenesis.23 In zebrafish, vegfd is expressed in tail bud and its overexpression can trigger aberrant sprouting of intersegmental arteries ,24 but its role in hematopoiesis has not yet been reported.
In this study, we show that Ncor2 regulates HSC development through fos-vegfd cascade, in which Ncor2 cooperates with Hdac3 to repress fos transcription during HSC development. When ncor2 is knocked down, fos will be upregulated, which then induces the expression of vegfd to repress HSC emergence by increasing Notch signaling activity, consequently.
Methods
Zebrafish lines
Adult zebrafish including the wild-type (AB strain), the transgenic line fli1:eGFP (generously provided by S. Wilson),25 and kdrl:mCherry/cmyb:GFP (generously provided by A. Meng),26 were raised and kept at 28.5°C in the standard circulating water system. Zebrafish embryos were acquired by natural spawning. This study was approved by the Ethical Review Committee of the Institute of Zoology, Chinese Academy of Sciences, Beijing, China.
WISH
Whole mount in situ hybridization (WISH) of zebrafish embryos was performed as described previously27 using probes for ncor2, runx1, cmyb, rag1, fos, dll4, ephrinB2, vegfd, gata1, l-plastin, pu.1, aldh1a2, and cyp26a1. The embryos were observed with a Nikon C-DSS230 stereo-microscope, and the images were taken with a Nikon DS-U2 camera using NIS-Elements (version F3.0).
MOs, mRNA synthesis, and microinjection
All morpholinos (MOs) were ordered from Gene Tools and dissolved with distilled H2O into 1 mM as stock solutions. The MOs sequences are listed in supplemental information (see supplemental Table 1 on the Blood Web site). \MO evaluation is described in the supplemental Methods For messenger RNA (mRNA) synthesis, zebrafish fos and vegfd full-length cDNA synthesis (CDS) were cloned into the pCS2 vector with BamH I and Xho I. Capped mRNA was synthesized using the mMESSAGE mMACHINE SP6 Kit (Ambion) and purified by a RNA purification kit (Tiangen). MOs and mRNAs were injected into 1-cell stage zebrafish embryos at the yolk/blastomere boundary. For overexpression of fos or vegfd specifically in the blood vessel region, the full-length CDS of zebrafish fos or vegfd was cloned into pDONR221 vector by BP reaction, then subcloned into a vector with a fli1 promoter and a GFP reporter by LR reaction (MultiSite Gateway Technology; Invitrogen).
Generation of ncor2 mutant, quantitative reverse-transcription polymerase chain reaction (qRT-PCR), TUNEL, and BrdU assay
The detailed protocols for these assays are described in the supplemental Methods. The primer information is listed in supplemental Tables 2, 4, and 5).
Chemical treatment
Zebrafish embryos were incubated with 0.1 μM Trichostatin A (TSA; [R-(E,E)]-7-[4-(Dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide; Sigma-Aldrich) at 10 hpf, or with 5 to 10 μM Notch inhibitor (DAPT) (LY-374973 N-[N-(3,5-Difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester; Sigma-Aldrich) at 24 hpf, which were harvested at 36 hpf. Those incubated in dimethylsulfoxide (DMSO), the dilution ratio is in line with that of the examined chemical, were served as the control.
ChIP assay
Chromatin immunoprecipitation (ChIP) assay was carried out with the control or ncor2 morphant embryos at 26 hpf, and the eluted DNA which was precipitated by anti-H3K27Ac (Abcam) and anti-H3K9Ac (Millipore) or rabbit purified IgG (negative control), was assayed by quantitative polymerase chain reaction (qPCR), as previously described.28 The PCR primers are listed in supplemental Table 3.
Western blot
The trunks of zebrafish embryos were dissected and homogenized with cell lysis buffer (protein inhibitor was added). The protein concentration of the lysate was quantified by Bradford protein assay. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred into a nitrocellulose membrane. After blocking by nonfat milk, the membrane was incubated with anti-Runx1 (1:150; AnaSpec) or anti-Ncor2 (1:250; Abcam) antibody diluted in blocking buffer at 4°C overnight, then incubated with an alkaline phosphatase conjugated second antibody (1:5000; Jackson ImmunoResearch Laboratories) for 3 hours at room temperature. Finally, the membrane was washed and the signal was assayed with a chemiluminescent horseradish peroxidase substrate (Millipore).
Confocal microscopy
Zebrafish embryos were scanned by a Zeiss LSM 510 META confocal laser microscope, and the images were generated by 3D projections with the Zeiss LSM software (Carl Zeiss).28
Statistical analysis
For statistical analysis, Student unpaired 2-tailed t test was used for all comparisons.
Results
ncor2 is expressed in artery and necessary for HSC development
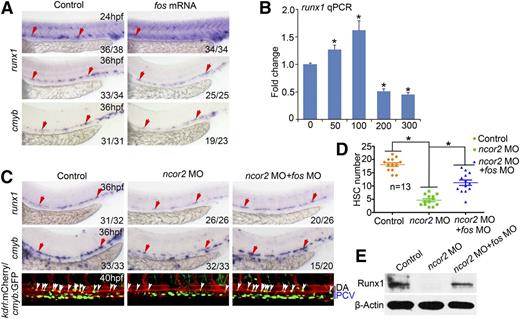
In the early development of zebrafish embryos, ncor2 was expressed in the aorta-gonad-mesonephros (AGM) region at 24 hpf (Figure 1A), suggesting its potential involvement in vasculogenesis or hematopoiesis. We also detected ncor2 expression in endothelial cells and HSCs in the AGM by double fluorescence in situ hybridization (FISH) (Figure 1B). To further validate this observation, we sorted hematopoietic cells (cmyb:GFP positive cells) or hematopoietic and hemogenic endothelial cells (gfi1:GFP positive cells).29,30 Gene expression analysis using qRT-PCR revealed that ncor2 was significantly enriched in these hematopoietic and hemogenic endothelial cells (supplemental Figure 1A-B; P < .05). To explore the role of ncor2 during HSC development, the previously reported splice MO was used to knockdown ncor2.15 The specificity of this MO was validated by western blot (Figure 1C), RT-PCR, and sequencing (supplemental Figure 1C-E). WISH result demonstrated that, in ncor2 morphants, HSC marker runx1 was severely reduced in the AGM region at 26 and 36 hpf, whereas cmyb was significantly reduced in the AGM at 36 hpf and in the caudal hematopoietic tissue (CHT) region at 4 dpf (Figure 1D). Interestingly, T-cell marker rag1 was even absent in the thymus at 4 dpf (Figure 1D). kdrl:mCherry/cmyb:GFP transgenic line was employed to visualize the HSC population in the AGM region. The number of kdrl+/cmyb+ double-positive cells in the AGM region (indicated by white arrows, or emerging HSCs), was remarkably reduced in ncor2 morphants (Figure 1E-F). Western blot analysis also revealed that Runx1 was almost undetectable in ncor2 morphants at both 24 and 36 hpf (Figure 1G). The decreased expression of runx1 and rag1 was further confirmed by an atgMO, as well as an ncor2 mutant generated by CRISPR/Cas9 method (with 8 bp deletion in the coding region of ncor2) (supplemental Figure 2A-D), demonstrating that the observed HSC defects in ncor2-deficient embryos were specific. In addition, gata1 (erythroid), l-plastin, and pu.1 (myeloid) were all decreased in the CHT region at 4 dpf (supplemental Figure 3A). Insofar as most hematopoietic cells in the CHT or the thymus are derived from the AGM region, the defect in thymus and CHT may result from the blocked HSC development in the AGM region.
ncor2 is expressed in the AGM region and is required for HSC development in zebrafish. (A) Cryosectioning of zebrafish embryos after WISH demonstrated that ncor2 is expressed in the blood vessel at 24 hpf. (B) Double FISH revealed that ncor2 is coexpressed with fli1 and cmyb in the AGM region. (C) Western blot revealed the protein level of Ncor2 in the control and ncor2 morphants at 24 hpf. (D) The expression pattern of HSC markers (runx1, cmyb) in the AGM region at 26 and 36 hpf, in the CHT region, and the T-cell marker (rag1) in the thymus at 4 dpf. (E) Confocal images using kdrl:mCherry/cmyb:GFP line demonstrated the HSC number in the control and ncor2 morphants at 36 hpf. White arrows indicate HSCs in the AGM region. (F) The quantification of kdrl+/cmyb+ HSCs shown in (E) (mean ± standard error of the mean [SEM], n = 12, *P < .05). (G) Western blot showed the protein level of Runx1 in the control and ncor2 morphants at 24 and 36 hpf. DA, dorsal aorta; PCV, posterior cardinal vein.
ncor2 is expressed in the AGM region and is required for HSC development in zebrafish. (A) Cryosectioning of zebrafish embryos after WISH demonstrated that ncor2 is expressed in the blood vessel at 24 hpf. (B) Double FISH revealed that ncor2 is coexpressed with fli1 and cmyb in the AGM region. (C) Western blot revealed the protein level of Ncor2 in the control and ncor2 morphants at 24 hpf. (D) The expression pattern of HSC markers (runx1, cmyb) in the AGM region at 26 and 36 hpf, in the CHT region, and the T-cell marker (rag1) in the thymus at 4 dpf. (E) Confocal images using kdrl:mCherry/cmyb:GFP line demonstrated the HSC number in the control and ncor2 morphants at 36 hpf. White arrows indicate HSCs in the AGM region. (F) The quantification of kdrl+/cmyb+ HSCs shown in (E) (mean ± standard error of the mean [SEM], n = 12, *P < .05). (G) Western blot showed the protein level of Runx1 in the control and ncor2 morphants at 24 and 36 hpf. DA, dorsal aorta; PCV, posterior cardinal vein.
Given that Ncor2 can bind to the retinoic receptor to repress retinoic acid (RA) signaling, we examined the expression of aldh1a2 and cyp26a1 by WISH, which is required for RA metabolism, and thus can indicate the level of RA in zebrafish.31 Our results indicated that, in ncor2 morphants, aldh1a2 was not significantly changed, whereas the slight increase of cyp26a1 was mainly restricted in the tail bud but not the AGM region (supplemental Figure 3B-C), suggesting that RA is not responsible for the HSC defects in ncor2 morphants (ncor1 morphants was taken as the control).
MO injection could lead to a p53-dependent apoptosis.32 To exclude this possibility, we coinjected zebrafish embryos with ncor2 and p53 MOs. WISH results revealed that expression of runx1 was significantly decreased in those coinjected embryos (supplemental Figure 4A), suggesting that the decrease of HSCs was caused specifically by ncor2 MO knockdown. To further investigate whether the decrease of HSCs in ncor2 morphants could be attributed to the alteration of the proliferation and apoptosis rate, we employed BrdU and TUNEL assay, respectively. The results indicated that no significant alteration in proliferation was observed in ncor2 morphants (supplemental Figure 4B). On the other hand, apoptosis signal was slightly enhanced, which primarily occurred in the neural tube; barely no signal was observed in the blood vessel region both in the control and ncor2 morphants (supplemental Figure 4C). In line with the result of the TUNEL assay, time-lapse microscopy with Tg(cmyb:GFP/kdrl:mCherry) embryos from 32 to 42 hpf showed that the specification, but not apoptosis, of cmyb:GFP positive cells was affected in ncor2 morphants (supplemental Video 1-2). Thus, the decrease of HSCs in ncor2 morphants could not be explained by alteration in proliferation or apoptosis.
Ncor2 represses fos expression in cooperation with Hdac3
Ncor2 acts as a corepressor in gene transcription.12,33 To screen the downstream factors of Ncor2, a number of genes that have been implicated in HSC formation were examined systematically in ncor2 morphants (data not shown). For the upregulated genes, we tried to rescue the decrease of runx1 expression by coinjection of the corresponding MOs (fos, vegfd, vegfa, vegfc, erk2, and mef2) or treatment using chemical inhibitors (PD98059). However, we failed to observe any effective rescue (data not shown) with these manipulations, except fos MO and vegfd MO. Therefore, fos was chosen due to its role in HSC development19,20 and highly increased expression in the AGM region of ncor2 morphants (Figure 2A), which was also confirmed in embryos injected with ncor2 atgMO or ncor2 mutant (supplemental Figure 2B,D). Expression of fos was also verified by qPCR (Figure 2B). Given that Ncor2 usually tethers Hdacs to conduct the transcriptional repression, hdac1 and hdac3 MOs were designed. WISH result revealed that knockdown hdac3, but not hdac1, caused the enhanced expression of fos in the AGM region (Figure 2C and data not shown). Moreover, hdac3 MO injection or Hdacs inhibitor TSA treatment also caused the decrease of runx1 expression (Figure 2C). Double FISH revealed that ncor2 is colocalized with hdac3 in the blood vessels in the AGM region (Figure 2D). These data suggest that Ncor2 may regulate HSC development through interacting with Hdac3 to repress fos expression. To test this hypothesis, we performed ChIP with antibodies against H3K9Ac and H3K27Ac, which are commonly used to mark active gene promoters and enhancers. The ChIP result demonstrated that the acetylation level (H3K9Ac and H3K27Ac) in the promoter of fos was enhanced (Figure 2E), which is in line with the increased fos expression in ncor2 morphants (Figure 2A-B). The enhanced acetylation state was also observed in hdac3 morphants and in the embryos co-injected with low doses of ncor2 MO and hdac3 MO (Figure 2E). These results strongly suggest that Ncor2 may repress fos expression by deacetylating its promoter in cooperation with Hdac3.
Ncor2 represses fos expression in cooperation with Hdac3. (A) The expression pattern of fos in the control or ncor2 morphants at 26 and 36 hpf. (B) qPCR assay revealing the expression level of fos in the control or ncor2 morphants at 26 and 36 hpf (mean ± standard deviation [SD], n = 3, *P < .05). (C) The expression pattern of fos in hdac3 morphants and runx1 in hdac3 morphants or TSA-treated embryos. (D) FISH result indicated that ncor2 is coexpressed with hdac3 in the blood vessel region. (E) ChIP assay. The diagram depicts the location of primers used in the ChIP assay (upper panel). The pair of acetylation detection primers (fos-ac-1F/R) spans the transcription start site whereas the control pair (fos-ac-CF/R) is located about 700 bp upstream of the transcription start site. The ChIP result showed the acetylation state of the hyper-acetylation region (fos-ac-1F/R) and the upstream control region (fos-ac-CF/R) of the fos promoter (mean ± SD, n = 3, *P < .05) in ncor2 morphants, hdac3 morphants, and embryos coinjected with a low dose of ncor2 and hdac3 MO (lower panel), respectively.
Ncor2 represses fos expression in cooperation with Hdac3. (A) The expression pattern of fos in the control or ncor2 morphants at 26 and 36 hpf. (B) qPCR assay revealing the expression level of fos in the control or ncor2 morphants at 26 and 36 hpf (mean ± standard deviation [SD], n = 3, *P < .05). (C) The expression pattern of fos in hdac3 morphants and runx1 in hdac3 morphants or TSA-treated embryos. (D) FISH result indicated that ncor2 is coexpressed with hdac3 in the blood vessel region. (E) ChIP assay. The diagram depicts the location of primers used in the ChIP assay (upper panel). The pair of acetylation detection primers (fos-ac-1F/R) spans the transcription start site whereas the control pair (fos-ac-CF/R) is located about 700 bp upstream of the transcription start site. The ChIP result showed the acetylation state of the hyper-acetylation region (fos-ac-1F/R) and the upstream control region (fos-ac-CF/R) of the fos promoter (mean ± SD, n = 3, *P < .05) in ncor2 morphants, hdac3 morphants, and embryos coinjected with a low dose of ncor2 and hdac3 MO (lower panel), respectively.
fos acts downstream of ncor2 to regulate HSC development
To demonstrate the link between fos aberrant expression and HSC phenotypes, we injected zebrafish embryos with fos mRNA and found that a high dose (200 pg) of fos led to the decreased expression of HSC markers runx1 and cmyb (Figure 3A). Decreased runx1 expression was also confirmed by qPCR (Figure 3B). According to the qPCR and the WISH results, we observed that a low level of fos mRNA led to an increase of runx1 expression whereas a high level of fos caused the opposite effect (Figure 3B and supplemental Figure 5A). To further investigate if Fos specifically affects HSCs through functioning in neighboring endothelial cells, we constructed a fusion plasmid in which the fos coding sequence (CDS) was fused with a GFP reporter driven by fli1 promoter (fli1-ep-fos-gfp). We co-injected zebrafish embryos with this plasmid and tol2 mRNA, and embryos with GFP expression specifically in the blood vessels which were subjected to WISH. The result demonstrated that runx1 was increased in embryos with a low level of GFP signal whereas it significantly decreased in those with a high level of GFP signal (supplemental Figure 5B). These findings indicated that fos expression needs to be maintained at an appropriate level for HSC emergence.
fos acts downstream of ncor2 to regulate HSC development. (A) The expression level of runx1 and cmyb in embryos injected with fos mRNA at 24 and 36 hpf. (B) qPCR analysis of runx1 expression within embryos injected with a series of doses of fos mRNA (mean ± SD, n = 3, *P < .05). (C) WISH result showed the expression level of runx1 or cmyb in the AGM region at 36 hpf (top and middle panels). The HSCs number in the control, ncor2 MO injected, ncor2 and fos MOs coinjected embryos at 40 hpf, within the kdrl:mCherry/cmyb:GFP line (bottom panel). White arrows indicate HSCs in the AGM region. (D) The quantification of kdrl+/cmyb+ HSCs shown in (C) (mean ± SEM, n = 13, *P < .05). (E) Western blot demonstrated protein level of Runx1 in the control, ncor2 MO injected, ncor2 and fos MOs coinjected embryos at 36 hpf.
fos acts downstream of ncor2 to regulate HSC development. (A) The expression level of runx1 and cmyb in embryos injected with fos mRNA at 24 and 36 hpf. (B) qPCR analysis of runx1 expression within embryos injected with a series of doses of fos mRNA (mean ± SD, n = 3, *P < .05). (C) WISH result showed the expression level of runx1 or cmyb in the AGM region at 36 hpf (top and middle panels). The HSCs number in the control, ncor2 MO injected, ncor2 and fos MOs coinjected embryos at 40 hpf, within the kdrl:mCherry/cmyb:GFP line (bottom panel). White arrows indicate HSCs in the AGM region. (D) The quantification of kdrl+/cmyb+ HSCs shown in (C) (mean ± SEM, n = 13, *P < .05). (E) Western blot demonstrated protein level of Runx1 in the control, ncor2 MO injected, ncor2 and fos MOs coinjected embryos at 36 hpf.
To elucidate the role of fos upregulation in ncor2 morphants, an atgMO of fos was designed. In the embryos coinjected with pEGFPN1-fos and fos atgMO, the GFP signal was markedly inhibited, indicating that fos atgMO worked efficiently (supplemental Figure 5C). Then, we performed a double knockdown by co-injection of ncor2 and fos MOs into 1-cell stage embryos. The WISH result showed that an optimal dose of fos (1 ng) MO partially rescued the HSC defects in ncor2 morphants (Figure 3C). To further support this data, the kdrl:mCherry/cmyb:GFP line was used. Our results clearly showed that very few kdrl+/cmyb+ cells were observed in the AGM region of ncor2 morphants, whereas more GFP+ cells were found in the corresponding region when embryos were coinjected with ncor2 and fos MOs (Figure 3C-D). This rescue effect was further confirmed by western blotting (Figure 3E). Taken together, these results demonstrated that fos functions downstream of ncor2 to mediate HSC emergence.
Arterial fate is promoted in ncor2 morphants and fos overexpressed embryos
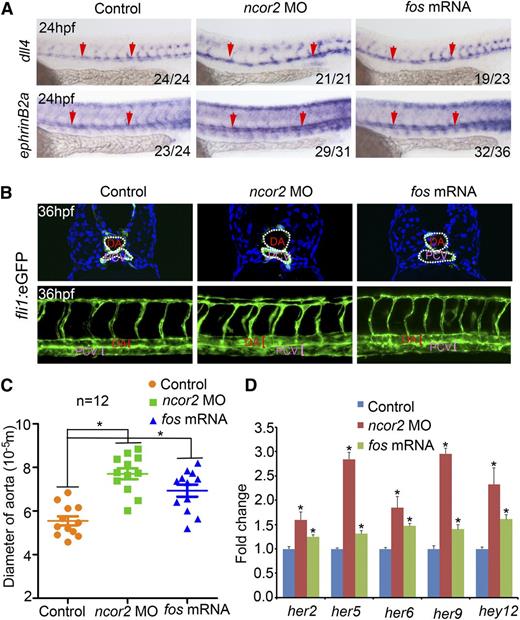
Because HSCs are derived from the embryonic dorsal aorta in vertebrates,34 we examined the expression of arterial markers in ncor2 morphants and fos overexpressed embryos. The increased expression of arterial marker dll4 and ephrinB2a was detected in ncor2 morphants and fos overexpressed embryos (Figure 4A). Furthermore, we performed cryosectioning and confocal microscopy using the fil1:eGFP embryos. The enlarged artery was observed in ncor2 morphants and fos overexpressed embryos (Figure 4B-C), which indicated that the arterial fate may be enhanced when ncor2 was knocked down. Because dll4 and ephrinB2a are components of the Notch pathway, expression of a number of additional Notch target genes including her2, her5, her6, her9, and hey12 was examined. Our results indicated that the expression of these Notch target genes was increased in ncor2 morphants and fos overexpressed embryos (Figure 4D). Taken together, ncor2 may inhibit the arterial fate by repression of fos, which acts as a potential positive regulator of Notch signaling.
Arterial identity and Notch signal are enhanced in ncor2 morphants and fos overexpressed embryos. (A) The expression pattern of dll4 and ephrinB2a in the control, ncor2 morphants, and fos-overexpressed embryos at 24 hpf. (B) Cryosectioning and confocal imaging revealed the enlarged artery in ncor2 morphants and fos-overexpressed embryos at 36 hpf, compared with the controls. (C) The quantification of the diameter of the dorsal aorta shown in (B) (mean ± SEM, n = 12, *P < .05). (D) qPCR result demonstrated Notch targets her2, her5, her6, her9, and hey12 were increased in ncor2 morphants and fos-overexpressed embryos at 24 hpf. (mean ± SD, n = 3, *P < .05).
Arterial identity and Notch signal are enhanced in ncor2 morphants and fos overexpressed embryos. (A) The expression pattern of dll4 and ephrinB2a in the control, ncor2 morphants, and fos-overexpressed embryos at 24 hpf. (B) Cryosectioning and confocal imaging revealed the enlarged artery in ncor2 morphants and fos-overexpressed embryos at 36 hpf, compared with the controls. (C) The quantification of the diameter of the dorsal aorta shown in (B) (mean ± SEM, n = 12, *P < .05). (D) qPCR result demonstrated Notch targets her2, her5, her6, her9, and hey12 were increased in ncor2 morphants and fos-overexpressed embryos at 24 hpf. (mean ± SD, n = 3, *P < .05).
fos activation induces vegfd and then leads to HSC defects
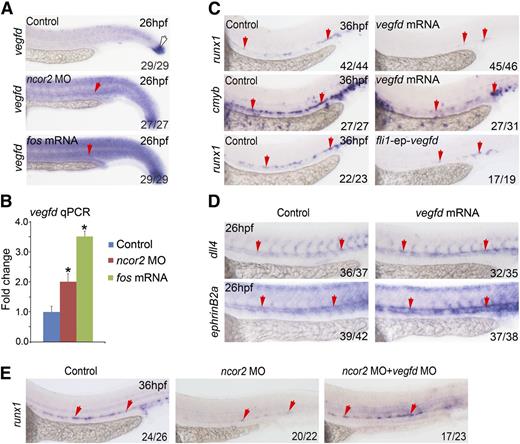
It is known that vegfd can be induced by fos expression in endothelial cells.35 Using WISH, we observed that overexpression of fos or knocking down ncor2 resulted in increased vegfd expression, including the AGM region (Figure 5A, red arrows). Similar results were also obtained using qPCR (Figure 5B). Importantly, this result was also confirmed in embryos injected with ncor2 atgMO or the genetic mutant (supplemental Figure 2B,D). To determine whether vegfd is involved in HSC development, we overexpressed vegfd mRNA in zebrafish embryos and then analyzed the expression of HSC markers using WISH. The expression of runx1 and cmyb was markedly decreased in the AGM region at 36 hpf (Figure 5C). To further confirm that vegfd functions in endothelial cells, we co-injected 1-cell stage zebrafish embryos with fli1-ep-vegfd-gfp together with tol2 mRNA, and embryos with GFP expression specifically in the blood vessels were subjected to WISH. The result demonstrated that the expression of runx1 was significantly reduced in fli1-ep-vegfd-gfp injected embryos (Figure 5C), which is in line with the observation for vegfd mRNA injection experiments. We also checked the expression of arterial markers in vegfd overexpressed embryos. Similar to the ncor2 morphants, the expression of dll4 and ephrinB2a was increased (Figure 5D). As a result, overexpression of fos induced expression of vegfd, which then led to the increased expression of arterial markers, and consequently, conferring the observed HSC defects. The double knockdown of both ncor2 and vegfd further confirmed that increased vegfd expression is responsible for the HSC defects in ncor2 morphants (Figure 5E). Taken together, in ncor2 morphants, fos-induced vegfd expression plays a negative role during HSC formation.
vegfd functions downstream of nocr2 and fos to repress HSC emergence. (A) The expression pattern of vegfd in the control, ncor2 morphants, and fos overexpressed embryos at 26 hpf. Red arrows mark the AGM region, whereas white arrow marks the tail bud. (B) qPCR result showed the expression level of vegfd in the control, ncor2 morphants, and fos-overexpressed embryos at 26 hpf (mean ± SD, n = 3, *P < .05). (C) The expression pattern of runx1 and cmyb in the control and vegfd-overexpressed embryos at 36 hpf (top and middle panels). The expression level of runx1 in the control or embryos coinjected with the fli1-ep-vegfd-gfp plasmid and tol2 mRNA (bottom panel). (D) The expression pattern of dll4 and ephrinB2a in the control and vegfd-overexpressed embryos. (E) Knocking down vegfd could partially rescue the decrease of runx1 in ncor2 morphants.
vegfd functions downstream of nocr2 and fos to repress HSC emergence. (A) The expression pattern of vegfd in the control, ncor2 morphants, and fos overexpressed embryos at 26 hpf. Red arrows mark the AGM region, whereas white arrow marks the tail bud. (B) qPCR result showed the expression level of vegfd in the control, ncor2 morphants, and fos-overexpressed embryos at 26 hpf (mean ± SD, n = 3, *P < .05). (C) The expression pattern of runx1 and cmyb in the control and vegfd-overexpressed embryos at 36 hpf (top and middle panels). The expression level of runx1 in the control or embryos coinjected with the fli1-ep-vegfd-gfp plasmid and tol2 mRNA (bottom panel). (D) The expression pattern of dll4 and ephrinB2a in the control and vegfd-overexpressed embryos. (E) Knocking down vegfd could partially rescue the decrease of runx1 in ncor2 morphants.
The decrease of HSCs in ncor2 morphants, fos or vegfd overexpressed embryos can be partially rescued by blocking Notch signaling
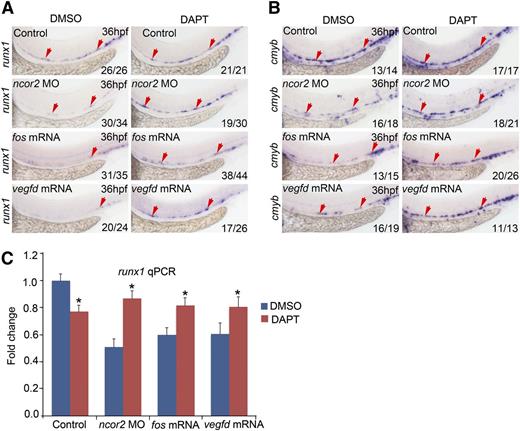
Transient downregulation of Notch signaling is necessary for runx1 expression at the onset of definitive hematopoiesis in chicken embryos.36 In mouse embryos, sustained activation of Notch1 in VE-cadherin expressing endothelial cells resulted in the absence of intra-aortic clusters.37 These experiments suggest that the increased expression of dll4 and ephrinB2a in the artery of ncor2 morphants may inhibit HSC emergence. Therefore, we treated the ncor2 morphants with DAPT, a chemical inhibitor of Notch signaling, from 24 hpf (after artery/vein specification) to 36 hpf. The results indicated that treatment with DAPT partially rescued the decreased expression of runx1 and cmyb in ncor2 morphants and fos or vegfd overexpressed embryos (Figure 6A-B). Gene expression using qPCR indicated that the result was consistent with the WISH result (Figure 6C). Together, the HSC decrease in ncor2 morphants might be attributed to the enhanced Notch signaling.
Inhibition of Notch signal can partially rescue the decrease of HSCs in ncor2 morphants and vegfd-overexpressed embryos. (A) The expression pattern of runx1 in the control, ncor2 morphants, fos-overexpressed embryos, and vegfd-overexpressed embryos at 36 hpf, which were treated with DMSO or with a γ-secretase inhibitor DAPT from 24 to 36 hpf. (B) The expression pattern of cmyb in the control, ncor2 morphants, fos-overexpressed embryos, and vegfd-overexpressed embryos at 36 hpf, which were treated with DMSO or with DAPT from 24 to 36 hpf. (C) qPCR result showing the expression level of runx1 in the control, ncor2 morphants, fos-overexpressed embryos, and vegfd-overexpressed embryos at 36 hpf, which were treated with DMSO or with DAPT from 24 to 36 hpf (mean ± SD, n = 3, *P < .05).
Inhibition of Notch signal can partially rescue the decrease of HSCs in ncor2 morphants and vegfd-overexpressed embryos. (A) The expression pattern of runx1 in the control, ncor2 morphants, fos-overexpressed embryos, and vegfd-overexpressed embryos at 36 hpf, which were treated with DMSO or with a γ-secretase inhibitor DAPT from 24 to 36 hpf. (B) The expression pattern of cmyb in the control, ncor2 morphants, fos-overexpressed embryos, and vegfd-overexpressed embryos at 36 hpf, which were treated with DMSO or with DAPT from 24 to 36 hpf. (C) qPCR result showing the expression level of runx1 in the control, ncor2 morphants, fos-overexpressed embryos, and vegfd-overexpressed embryos at 36 hpf, which were treated with DMSO or with DAPT from 24 to 36 hpf (mean ± SD, n = 3, *P < .05).
Discussion
It is well accepted that HSCs are specified from vascular endothelial cells through a transition of hemogenic endothelial cells.5-7,34 However, the detailed underlying molecular mechanisms are largely unknown. Here, we show that ncor2 knockdown upregulates expression of fos, and then vegfd, to enhance the arterial fate via Notch signaling, which unexpectedly inhibits the hemogenic endothelium formation, consequently resulting in the failure of HSC emergence in the AGM region.
ncor2 was detected in the AGM region at 24 hpf during zebrafish embryogenesis, when the hemogenic endothelium, marked by runx1 expression, began to be specified. The observation of decreased expression of HSC markers in ncor2 morphants from 26 hpf suggests that Ncor functions at the time when hemogenic endothelium forms, which is well consistent with its expression at this stage. The increased expression of arterial markers and enlarged artery lumen in ncor2 morphants further revealed that the arterial fate was altered, which might be not appropriate for HSC development. As a member of the Ncor complex, Hdacs are a group of pivotal enzymes that erase the histone acetylation state in promoters of their targets to execute transcriptional repression. When Hdacs were blocked by TSA in neurons, the expression of fos was induced.22 We also found that knocking down hdac3 or ncor2 led to the increased expression of fos, which is required for HSC proliferation.17,18 However, whether fos is involved in HSC emergence is unknown. Injection of fos mRNA in zebrafish embryos altered the expression of runx1 in a dose-dependent manner, that is, fos promotes the expression of runx1 at a lower dose and inhibits it at a higher dose (supplemental Figure 5A-B). Thus, the HSC defects observed in ncor2 morphants may be attributed to the ectopic induction of fos. But how fos regulates runx1 expression has not been reported yet. Vegfd is a vascular growth factor and it can be induced by fos.35 Although the effect of vegfd overexpression on angiogenesis has been reported in zebrafish,24 its role in hematopoiesis is unclear. Overexpression of vegfd mRNA in zebrafish embryos caused the decreased expression of HSC markers similar to ncor2 morphants. We also observed increased expression of dll4 in the vegfd overexpressed embryos, which is consistent with the previous report that vegfd can activate the expression of dll4.38 As HSCs are derived from the hemogenic endothelium, how these hemogenic endothelial cells lose their endothelial identity and acquire a hematopoietic fate remains elusive. A very recent study reported that downregulation of the Notch pathway is a prerequisite to initiating the expression of runx1 during definitive hematopoiesis in chicken embryos,36 indicating that Notch signaling should be inhibited during the EHT process. In our study, we treated ncor2 morphants, fos, or vegfd overexpressed embryos with DAPT, and found that the decreased expression of runx1 in these embryos was partially rescued, suggesting that Ncor2 negatively regulates Notch signaling via fos-vegfd cascade to tightly control HSC emergence. However, the underlying mechanisms of Notch inhibition at the onset of HSC development in vertebrates awaits further investigation.
ncor2 is expressed in the AGM region whereas fos and vegfd are not, indicating that fos and vegfd may be repressed in this region under normal condition. Although endogenous ncor2 was knocked down in zebrafish embryos, its repression of fos expression in the trunk was relieved, and therefore, fos was re-activated which then led to an increase of vegfd expression in this region ectopically. The balanced level of fos expression must be tightly controlled. Either upregulation or downregulation of fos expression will disturb the delicate balance, which is critical for normal HSC development. This is highly similar to the ERK signaling in the AGM region where its finely controlled threshold is crucial for HSC emergence in vertebrates.39 The dosage sensitive role of fos levels in HSC emergence is currently unclear. A recent report showed that c-Fos, plus 3 other transcription factors are sufficient to convert mouse fibroblasts into hemogenic endothelium,4 suggesting that c-Fos can promote HSC emergence. This study is consistent with our finding here that a low dose of fos mRNA increased the population of HSCs in zebrafish embryos; however, a higher dose of fos activated Vegfd/Notch signaling to promote the arterial fate, which is detrimental to the following hemogenic endothelium and HSC formation.
Ncor2 can bind to different nuclear receptors including the Notch pathway effectors CSL (named after CBF1, Su[H] and LAG-1),40 thyroid-related hormone receptor, RA receptor, and a series of transcription factors.41 RA is inhibitory for HSC self-renewal.42,43 However, blocking the RA signal pathway did not alter expression of HSC markers runx1 and cmyb in zebrafish embryos at 36 hpf.44 Whether excessive RA plays a role in HSC emergence is unknown. In our study, the observation that RA was not increased in the AGM region in ncor2 morphants, may rule out the possibility that Ncor2 function on HSCs potentially through RA signal pathway in zebrafish. Interestingly, a very recent report showed that RA signaling is required for HSC development in mouse AGM at E10.5,45 supporting its important role in mammals.
In conclusion, our work demonstrates that Ncor2 is required for HSC emergence through cooperating with Hdac3 to regulate fos and vegfd signaling, and consequently facilitates the hemogenic endothelial cells to lose their endothelial identity and establish hematopoietic capabilities.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the laboratory members Panpan Zhang, Xinyan Lu, and Yanyan Ding for helpful discussions and critical reading of the manuscript.
This work was supported by grants from the National Basic Research Program of China (2010CB945300 and 2011CB943900), the National Natural Science Foundation of China (31271570), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01010110).
Authorship
Contribution: Y.W. performed the research and wrote the paper; D.M., Y.G., C.Z., and L.W. performed the research; and F.L. conceived the research, analyzed the data, and wrote the paper. All authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Feng Liu, State Key Laboratory of Biomembrane and Membrane Biotechnology, Institute of Zoology, Chinese Academy of Sciences, 1 Beichen West Rd, Chaoyang District, Beijing 100101, China; e-mail: liuf@ioz.ac.cn.

![Figure 1. ncor2 is expressed in the AGM region and is required for HSC development in zebrafish. (A) Cryosectioning of zebrafish embryos after WISH demonstrated that ncor2 is expressed in the blood vessel at 24 hpf. (B) Double FISH revealed that ncor2 is coexpressed with fli1 and cmyb in the AGM region. (C) Western blot revealed the protein level of Ncor2 in the control and ncor2 morphants at 24 hpf. (D) The expression pattern of HSC markers (runx1, cmyb) in the AGM region at 26 and 36 hpf, in the CHT region, and the T-cell marker (rag1) in the thymus at 4 dpf. (E) Confocal images using kdrl:mCherry/cmyb:GFP line demonstrated the HSC number in the control and ncor2 morphants at 36 hpf. White arrows indicate HSCs in the AGM region. (F) The quantification of kdrl+/cmyb+ HSCs shown in (E) (mean ± standard error of the mean [SEM], n = 12, *P < .05). (G) Western blot showed the protein level of Runx1 in the control and ncor2 morphants at 24 and 36 hpf. DA, dorsal aorta; PCV, posterior cardinal vein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/10/10.1182_blood-2013-11-541391/4/m_1578f1.jpeg?Expires=1767714955&Signature=XGGKB9~zM09Fdb57pUxHDCcduVWl5AgxNtWshx9M0EGSP5ZglbUW80fu~-3TApunBYFHPPtsKtnN3937l9hrF8dcDGeKa9S22ZD~AdpI~YK4pBhyskumQ49vhOc~WL84w20eeseHvf3H4w5ceiQO~aALLoIE~YE3k1ojeGKIJyIkiLnXkJ0JXXeDD2KBMwrbCalN84Iq-JgbhQd1zbKOPKP~xwQkhoJhE-YRst2hheDxu0IQqsonO6fktBxZfbRo8TqcQcBNXjUICp5IH8QFln7EuwzH1oq4VNcOvD9dv~5IW0fHIqtgoVu6pbhfx9OkslIR8ZIxw8sZbBpZDxvMCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Ncor2 represses fos expression in cooperation with Hdac3. (A) The expression pattern of fos in the control or ncor2 morphants at 26 and 36 hpf. (B) qPCR assay revealing the expression level of fos in the control or ncor2 morphants at 26 and 36 hpf (mean ± standard deviation [SD], n = 3, *P < .05). (C) The expression pattern of fos in hdac3 morphants and runx1 in hdac3 morphants or TSA-treated embryos. (D) FISH result indicated that ncor2 is coexpressed with hdac3 in the blood vessel region. (E) ChIP assay. The diagram depicts the location of primers used in the ChIP assay (upper panel). The pair of acetylation detection primers (fos-ac-1F/R) spans the transcription start site whereas the control pair (fos-ac-CF/R) is located about 700 bp upstream of the transcription start site. The ChIP result showed the acetylation state of the hyper-acetylation region (fos-ac-1F/R) and the upstream control region (fos-ac-CF/R) of the fos promoter (mean ± SD, n = 3, *P < .05) in ncor2 morphants, hdac3 morphants, and embryos coinjected with a low dose of ncor2 and hdac3 MO (lower panel), respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/10/10.1182_blood-2013-11-541391/4/m_1578f2.jpeg?Expires=1767714955&Signature=YvyK9f0yisPfWlwMVIxHfv0grkbK2k1BdCUTG2sER4MAVqL9boQquTxmOQ1olPnNSdobFrg17y-TDm-lvEpFic0mPGdJYzYPvON9rq4n0VpDpBZPBPX3v6zf1Z0N~K0zzqQ5uiMRjf9CpTiq-tgxWZ-PDM~dveCzpwQzmAUKGhH~bT8qVtmO9vbRuvmofe4iwaxmFyU0fCVoDaqF84Q9HC34QI8n0kuRfWJXoUmlSgO1Dk-sxjyCg1DrGqHmfE3guzHO7DrxsA2v7Ev~johkqL28lY5-Te1CTvjy75k27WVMSWd-RArtUHnSr2kgryU3UKC4l4~ZAL~0d~U-DB-WCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal