In this issue of Blood, Shochat et al report mutations in receptors for interleukin-7 (IL-7) and thymic stromal lymphopoietin (TSLP), resulting in a novel dimerization mechanism that drives acute lymphoblastic leukemias.1
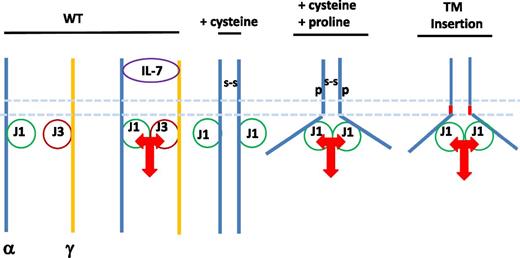
Model of ligand-independent signaling by mutant IL-7Rα: role of cysteine, proline, or transmembrane insertions. Hypothetical associations of IL-7Rα (α), γc, Jak1 (J1), Jak3 (J3), disulfide bond resulting from from cysteine insertions (S-S), proline insertions (P), and insertions in the transmembrane region (TM).
Model of ligand-independent signaling by mutant IL-7Rα: role of cysteine, proline, or transmembrane insertions. Hypothetical associations of IL-7Rα (α), γc, Jak1 (J1), Jak3 (J3), disulfide bond resulting from from cysteine insertions (S-S), proline insertions (P), and insertions in the transmembrane region (TM).
IL-7, produced by stromal cells in the thymus and other lymphoid organs, is required for thymic development and peripheral survival of T cells. The IL-7 receptor (IL-7R) consists of 2 chains, IL-7Rα and γc, which, via their intracellular domains, are associated with the Janus kinases Jak1 and 3, respectively. Genetic deficiency in IL-7Rα results in a critical lack of T cells, whereas polymorphisms in the gene are associated with multiple autoimmune diseases.2
T-cell acute lymphoblastic leukemia (T-ALL) is derived from thymocytes, and recently, several groups have reported gain-of-function mutations in IL-7Rα that act as drivers in ∼10% of pediatric T-ALL patients.3-5 The mutations generally consist of insertions into the region just outside the membrane and contain a cysteine and a few other amino acids. This aberrant presence of cysteine residues was shown to induce homodimerization of IL-7Rα and activation of Jak1, resulting in signaling that was independent of ligand, γc, or Jak3.
In the present study,1 the authors functionally assess several atypical mutations in IL-7Rα, ie, those that lacked cysteine insertions and were located at a more interior position, within the transmembrane region itself. They show that, like the mutations containing cysteines, the result is homodimerization, ligand-independent signaling, and leukemogenesis. It was surprising in the initial reports that homodimerization of the α chain (by any mechanism) induced signaling, because it had been shown that in this family of 2-chain receptors, cross-linking just 1 type of chain was insufficient to induce signaling.6 To explore this, the authors present modeling of the transmembrane region and suggest that the mutations, in addition to dimerizing the chains, may twist them, orienting the janus kinases of 2 α chains in proximity, allowing cross-phosphorylation and activation. This is reminiscent of an experiment (W.Q. Li, J. Barata, and S. Durum, unpublished data, 2011) with one of the cysteine insertion mutants, in which cysteine alone was sufficient to induce dimerization but not signaling. Furthermore, the presence of a proline residue, in addition to the cysteine, as in the original mutant, resulted in signaling (see figure). This could explain why no simple cysteine substitutions or insertions have been reported as yet.
The TSLP receptor is a different story. It shares the IL-7Rα chain, but uses CRLF2 as a second chain rather than γc. Unlike the insertions in IL-7Rα in T-ALL, overexpression of CRLF2 in B cell-derived acute lymphoblastic leukemia (B-ALL) is a frequent pattern that is created by chromosomal rearrangements.7-10 Overexpression confers a modest ligand-independent signal in vitro and may require TSLP in vivo to mediate its leukemic effects. CRLF2 can also display a gain-of-function mutation, F232C, that gives a stronger ligand-independent signal and is found in a subset of CRLF2 overexpressors in B-ALL. In the current report,1 a noncysteine mechanism is analyzed in mutations of CRLF2 in B-ALL. The study examined one such CLRF2 mutant, which, like the atypical IL-7Rα mutants, occurred within the transmembrane region and, in BaF3 cells induced homodimerization. This CRLF2 mutant required coexpression of IL-7Rα to signal and grow as leukemia in mice, suggesting it may also heterodimerize with IL-7Rα in an orientation that activates their associated Janus kinases.
These studies point to new leukemogenic signaling mechanisms and reinforce the IL-7/TSLP axis as therapeutic targets in ALL.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal