Key Points
The Optimul 96-well platelet aggregation assay has high levels of sensitivity and specificity for detecting platelet defects.
The requirement for a small volume of blood, straightforward nature, and speed make Optimul a promising screening test in bleeding patients.
Abstract
Up to 1% of the population have mild bleeding disorders, but these remain poorly characterized, particularly with regard to the roles of platelets. We have compared the usefulness of Optimul, a 96-well plate-based assay of 7 distinct pathways of platelet activation to characterize inherited platelet defects in comparison with light transmission aggregometry (LTA). Using Optimul and LTA, concentration-response curves were generated for arachidonic acid, ADP, collagen, epinephrine, Thrombin receptor activating-peptide, U46619, and ristocetin in samples from (1) healthy volunteers (n = 50), (2) healthy volunteers treated with antiplatelet agents in vitro (n = 10), and (3) patients with bleeding of unknown origin (n = 65). The assays gave concordant results in 82% of cases (κ = 0.62, P < .0001). Normal platelet function results were particularly predictive (sensitivity, 94%; negative predictive value, 91%), whereas a positive result was not always substantiated by LTA (specificity, 67%; positive predictive value, 77%). The Optimul assay was significantly more sensitive at characterizing defects in the thromboxane pathway, which presented with normal responses with LTA. The Optimul assay is sensitive to mild platelet defects, could be used as a rapid screening assay in patients presenting with bleeding symptoms, and detects changes in platelet function more readily than LTA. This trial was registered at www.isrctn.org as #ISRCTN 77951167.
Introduction
Mild bleeding disorders are prevalent in the general population with a frequency of up to 1%.1 Nonetheless, they often remain poorly characterized, both in terms of clinical and laboratory-based diagnosis.1 This stems in part from the fact that excessive bleeding only manifests in some patients in response to an appropriate challenge such as surgery, trauma, menstruation, and childbirth, and in part because the laboratory measures of platelet dysfunction, including the current gold standard light transmission aggregometry (LTA), require specialized expertise and are time-consuming.2 The recognition that bleeding is a multifactorial and complex process has fueled the search for platelet function assays that have the ability to assess a whole spectrum of platelet responses, require a small volume of blood, and require minimal technical expertise.2-4
Several attempts at developing a high-throughput platelet function assay are currently being investigated. These include devices that measure global platelet reactivity in response to shear alone,5 microfluidic devices with precoated adhesion or activation molecules,6-8 assays that measure calcium flux in platelets by fluorescent imaging,9 enzyme-linked immunosorbent assay–type assays to capture platelets on agonist-coated surfaces,10 luminometric assays of platelet secretion in response to various platelet agonists,11 and flow cytometric counting techniques with platelet immunostaining.12 Most of these techniques require specialized laboratory instruments (flow cytometers, osmotic pumps, microscopes, imaging devices) or can be expensive to perform (single use precoated cartridges or capillaries, fluorescent antibodies), and not all are sensitive to mild platelet inhibition,10 making them unsuitable for use in nonspecialized centers.
The development of the Optimul assay with lyophilized reagents on a standard half-area 96-well microtiter plate may provide a more cost-effective alternative for high-throughput platelet function testing.13 The assay requires a plate heater/shaker and an absorbance plate reader, which are available in most clinical and research laboratories.13 The Optimul assay is carried out on 2.5 mL of platelet-rich plasma (PRP), takes 10 minutes to perform and analyze, and provides full dose-response curves to 7 commonly used platelet agonists.13,14
We sought to investigate how the Optimul assay compares with the gold standard in platelet function testing, lumi-aggregomery, and whether it could be used to diagnose platelet defects in patients presenting with bleeding symptoms suggestive of inherited platelet function defects.
Material and methods
Participant selection
Healthy volunteers.
Participants were considered healthy if they were ≥18 years of age, did not require long-term medical therapy, had refrained from drugs known to influence platelet function in the previous 2 weeks, and did not have a history of bleeding symptoms.
Patients suffering from bleeding diathesis.
Participants with suspected inherited platelet function defects were recruited to the Genotyping and Phenotyping of Platelets study (GAPP; ISRCTN 77951167) from November 2011 to December 2012 from UK Comprehensive Care Haemophilia Centers and were invited to participate in this study if they satisfied all the following inclusion criteria: (1) abnormal bleeding symptoms compatible with a platelet function defect (spontaneous mucocutaneous bleeding or abnormal bleeding at other sites following trauma or invasive procedures); (2) results from coagulation factor tests within local laboratory reference intervals (minimum panel of prothrombin time, activated thromboplastin time, Clauss fibrinogen activity, von Willebrand profile); and (3) absence of demonstrable acquired platelet dysfunction. Patients with existing diagnoses of Glanzmann’s thrombasthenia, Bernard-Soulier syndrome, Hermansky Pudlak syndrome, or MYH9-related disorder were excluded. Participants with platelet counts outside the 150 to 450 × 109/L range were excluded. Laboratory testing was deferred in participants exposed within 2 weeks to drugs known to affect platelet function.
This study was approved by the National Research Ethics Service Committee West Midlands–Edgbaston (REC reference: 06/MRE07/36), and all participants gave written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Assessment of platelet function
Blood was drawn by venipuncture into evacuated tubes containing 3.1% trisodium citrate (S-Monovette 0.106 M; Sarstedt, Leicester, United Kingdom [UK], in line with the latest expert consensus documents.15 PRP was prepared by centrifugation of whole blood at 200g for 20 minutes. Platelet-poor plasma (PPP) was prepared by centrifugation of the remaining blood at 1000g for 10 minutes.
To assess the effect of antiplatelet drugs in vitro, in some experiments, samples were pretreated with 100 µM aspirin (Sigma-Aldrich, Dorset, UK) or 1 µM cangrelor (The Medicines Company, Abingdon, UK) prior to platelet function testing.
LTA.
Platelet function was assessed using LTA as described previously.16 Platelet aggregation was measured in PRP using a dual Chronolog lumiaggregometer (Model 460 VS; Havertown, PA) in response to ADP (Sigma-Aldrich, Poole, UK); epinephrine (Sigma-Aldrich, Poole, UK); arachidonic acid (AA; Cayman Chemical, Cambridge Bioscience Ltd.); PAR-1 receptor-specific peptide (SFLLRN; Alta Biosciences, Birmingham, UK), collagen (Nycomed, Linz, Austria); and ristocetin (Helena Biosciences, Sunderland, UK). ATP secretion was assessed using the Luciferin-Luciferase reagent (Chronolume) and an ATP standard solution. Results were classified as abnormal by reference to a bank of local healthy volunteers (presented in supplemental Figure 1 and supplemental Table 1, available on the Blood Web site).
Optimul assay.
Optimul 96-well plates were prepared as previously described.13 Briefly, flat-bottom half-area microtiter plates (Greiner Bio-One; Stonehouse, Gloucestershire, UK) were precoated with hydrogenated gelatin (0.75% weight/volume, Sigma-Aldrich, Poole, UK) in phosphate-buffered saline (Sigma-Aldrich, Poole, UK) to block the surface activation of platelets before the addition of platelet agonists. The agonists used were AA (0.03-1 mM; Sigma-Aldrich, Poole, UK), ADP (0.005-40 µM; Sigma-Aldrich, Poole, UK), epinephrine (0.0004-10 µM; Labmedics, Stockport, UK), collagen (0.01-40 µg/mL; Nycomed, Linz, Austria), TRAP-6 amide (SFLLRN; 0.03-40 µM, Bachem, St. Helens, UK), U46619 (0.005-40 µM; Labmedics, Stockport, UK), and ristocetin (0.14-4 mg/mL; Helena Biosciences, Tyne and Wear, UK). The agonists were lyophilized by placing the plates in a −80°C freezer for 1 hour and transferring into a freeze-dryer overnight at −40°C. The plates were then removed from the freeze-dryer, vacuum-sealed, foil-packed, and kept at room temperature until use within 12 weeks of manufacture.
PRP or PPP (40 µL) was added into the appropriate agonist-free control wells of the 96-well plate. PRP was then added to the agonist-coated wells with a multi-channel pipette. The plate was sealed with film and placed on a heater/shaker (BioShake iQ; Q Instruments, Jena, Germany) at 37°C to mix at 1200 rpm for 5 minutes. Absorbance was then measured at 595 nm on a 96-well plate reader (VersaMax Microplate reader; Associates of Cape Cod Inc., East Falmouth, MA). Platelet aggregation was expressed as the maximal percent change in light transmittance from PRP wells in response to agonists, using PPP wells as reference. Optimization and characterization of the Optimul assay have been previously reported.13,14,17
An Optimul panel was defined as abnormal if the dose-response curve was below the lower limit of the 95% reference interval for >1 agonist.
Plasma thromboxane B2.
After aggregation, indomethacin (30 µM) was added in relevant wells to stop cyclooxygenase (COX) activity. The whole plate was then centrifuged (1300g for 10 minutes) at room temperature, and the supernatants were collected and frozen at −80°C. Plasma thromboxane (Tx)B2 levels, a measure of TxA2 formation, were determined by a competitive immunoassay (in-house homogeneous time resolved fluorescence assay, developed with assistance of Cisbio Bioassays, Codolet, France).
Statistical analyses
Normally distributed continuous variables are presented as mean ± standard deviation, non-normally distributed continuous variables as median (interquartile range), and categorical variables as frequencies (percentages). Variables were analyzed for a normal distribution with the Kolmogorov-Smirnov test. Concentration-response curves were generated using a 4-parameter log-linear function in GraphPad Prism Software 5 for Windows (GraphPad Software, San Diego, CA). The area under the curve (AUC) analysis was carried out for each individual curve generated. For comparison of assays where the tests were performed by both assays on the same blood samples, paired analyses were used. Agreement in the classification of platelet defects between the 2 assays was assessed by the κ statistic. Analyses were performed with Statistical Package for the Social Sciences (SPSS) 14.0 for Windows (SPSS Institute).
Results
Optimul performance characteristics in healthy volunteers
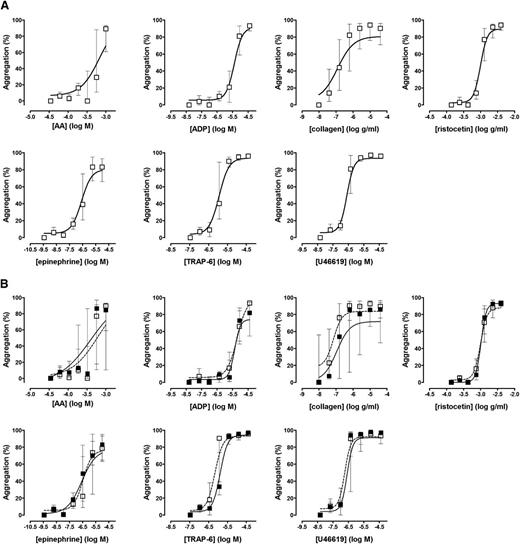
Platelet function was assessed in 50 healthy volunteers (aged 33 ± 7 years, 38% men); in 10 of these, platelet function testing was repeated 2 weeks later to assess intraindividual variability. As shown in Figure 1 and supplemental Table 2, with all agonists used in the Optimul assay, platelets displayed a dose-response relationship that was similar to that observed with LTA and was reproducible within subjects with repeat testing of blood sample obtained at different venepunctures. There was no association between the extent of platelet aggregation and either platelet count in PRP or mean platelet volume (supplemental Figure 2). We observed greater variability between healthy donor subjects in the Optimul test results compared with LTA test results at intermediate concentrations of AA and collagen (Figure 2). Because platelet aggregation responses to both collagen and AA are sensitive to defective metabolism of AA into TxA2, we investigated this difference further by measuring concentrations of TxB2 (a marker of TxA2 synthesis) in a subset of healthy volunteers (n = 25). This demonstrated that most healthy controls who displayed reduced aggregation responses by Optimul to AA and collagen also showed reduced TxB2 production (Figure 3), indicating that this group had impaired AA metabolism, most likely as a result of inadvertent nonsteroidal anti-inflammatory drug ingestion. Our observation that this group displayed normal LTA results with AA and collagen suggests that the Optimul offers greater sensitivity to defects in AA metabolism than LTA.
Reference intervals for the Optimul assay. (A) Dose-response curves in 50 healthy volunteers, presented as median and interquartile range. (B) Repeat testing in 10 healthy volunteers, presented as median and interquartile range. Open squares represent the first test, and closed squares represent repeat testing in the same individuals.
Reference intervals for the Optimul assay. (A) Dose-response curves in 50 healthy volunteers, presented as median and interquartile range. (B) Repeat testing in 10 healthy volunteers, presented as median and interquartile range. Open squares represent the first test, and closed squares represent repeat testing in the same individuals.
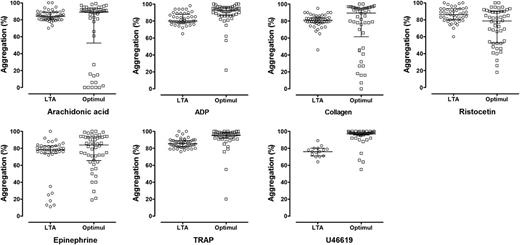
Agreement between LTA and the Optimul assay in healthy volunteers. Individual data for top concentrations of the agonists studied with LTA and within a similar range on Optimul: AA, 1 mM (LTA) to 1 mM (Optimul); ADP, 30-40 µM; collagen, 3 μg/mL to 2.5 µg/mL; epinephrine, 10 µM to 10 µM; TRAP, 100 µM peptide to 40 µM amide; U46619, 10 μM to 8.9 µM; ristocetin, 1.5 mg/mL to 1.3 mg/mL.
Agreement between LTA and the Optimul assay in healthy volunteers. Individual data for top concentrations of the agonists studied with LTA and within a similar range on Optimul: AA, 1 mM (LTA) to 1 mM (Optimul); ADP, 30-40 µM; collagen, 3 μg/mL to 2.5 µg/mL; epinephrine, 10 µM to 10 µM; TRAP, 100 µM peptide to 40 µM amide; U46619, 10 μM to 8.9 µM; ristocetin, 1.5 mg/mL to 1.3 mg/mL.
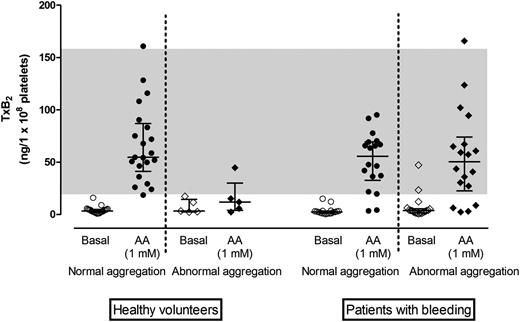
TxB2concentration in plasma supernatants from healthy volunteers and patients with bleeding symptoms. TxB2 concentration adjusted for platelet count in healthy volunteers with normal (n = 20) or impaired (n = 5) AA response on Optimul and in patients with bleeding disorders with normal (n = 18) or impaired (n = 17) AA response on Optimul. Line and whiskers represent median and interquartile range; the gray band represents the normal range derived from bank of healthy volunteers.
TxB2concentration in plasma supernatants from healthy volunteers and patients with bleeding symptoms. TxB2 concentration adjusted for platelet count in healthy volunteers with normal (n = 20) or impaired (n = 5) AA response on Optimul and in patients with bleeding disorders with normal (n = 18) or impaired (n = 17) AA response on Optimul. Line and whiskers represent median and interquartile range; the gray band represents the normal range derived from bank of healthy volunteers.
Effect of antiplatelet agents
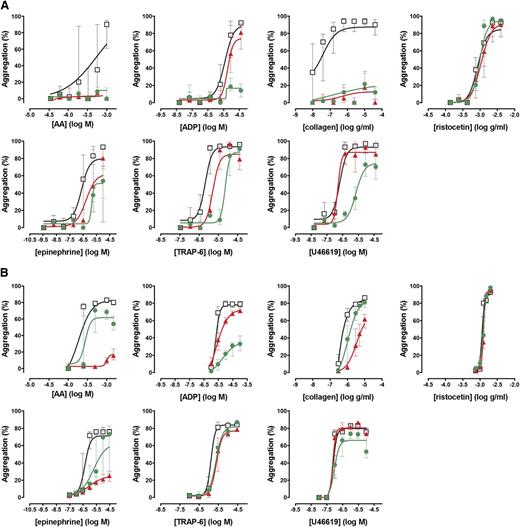
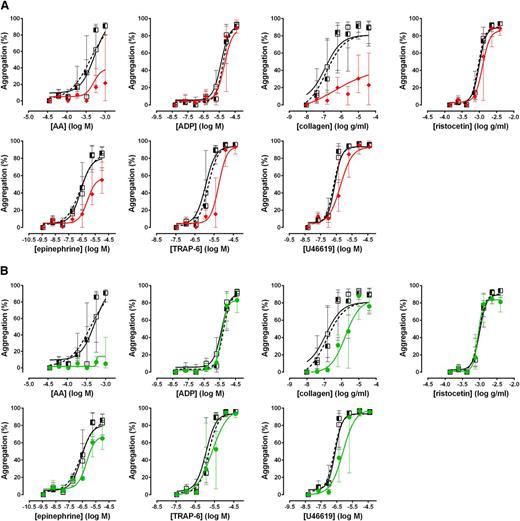
To assess the performance of the Optimul assay in detecting the effect of antiplatelet therapy, PRP samples obtained from 10 healthy volunteers were treated in vitro with 100 µM aspirin to assess COX inhibition or 1 µM cangrelor to assess P2Y12 receptor inhibition. As shown in Figure 4, both LTA and Optimul detected inhibition of platelet function by in vitro addition of antiplatelet agents, with Optimul being significantly more sensitive than LTA and displaying marked impairment in multiple platelet activation pathways, consistent with the role of TxA2 and ADP as amplifying signals for other agonists.18,19 Receiver-operator characteristic (ROC) curve analysis is presented in supplemental Figure 3, showing how the distribution of healthy donor response overlaps with healthy donor response treated with antiplatelet agents. Both lumi-aggregometry and the Optimul assay had high levels of discrimination, with Optimul achieving higher levels of AUC for multiple agonists (supplemental Figure 3). Most notably, inhibition of the P2Y12 receptor resulted in marked inhibition of AA- and collagen-induced platelet responses on Optimul that was not seen with LTA, highlighting the reliance of the Optimul assay on adequate ADP receptor activity as an amplifying signal leading to sustained platelet aggregation to these 2 agonists (Figure 4). Inhibition of COX by aspirin induced a less severe phenotype of platelet responses, with only AA- and collagen-induced aggregation being significantly affected (Figure 4).
Effect of antiplatelet agents added in vitro to samples from healthy volunteers on maximal platelet aggregation. (A) Dose-response curves obtained with Optimul in 10 healthy volunteers, presented as median and interquartile range. Open squares represent nontreated samples, the red triangles represent aspirin 100 µM–treated samples, and the green circles represent cangrelor 1 µM–treated samples in the same individuals. (B) Dose-response curves obtained with LTA in 10 healthy volunteers, presented as median and interquartile range. Open squares represent nontreated samples, the red triangles represent aspirin 100 µM–treated samples, and the green circles represent cangrelor 1 µM–treated samples in the same individuals.
Effect of antiplatelet agents added in vitro to samples from healthy volunteers on maximal platelet aggregation. (A) Dose-response curves obtained with Optimul in 10 healthy volunteers, presented as median and interquartile range. Open squares represent nontreated samples, the red triangles represent aspirin 100 µM–treated samples, and the green circles represent cangrelor 1 µM–treated samples in the same individuals. (B) Dose-response curves obtained with LTA in 10 healthy volunteers, presented as median and interquartile range. Open squares represent nontreated samples, the red triangles represent aspirin 100 µM–treated samples, and the green circles represent cangrelor 1 µM–treated samples in the same individuals.
Investigation of patients with a suspected impairment in platelet function
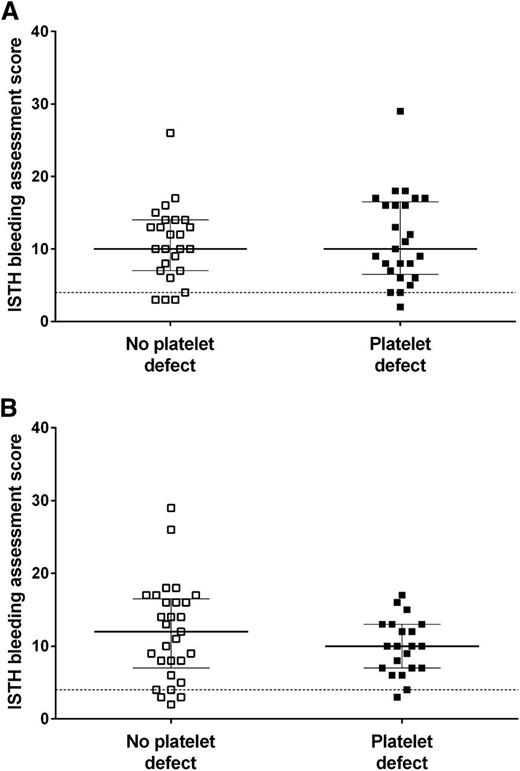
We sought to investigate whether the Optimul assay could be used to detect platelet defects in patients with a suspected inherited impairment in platelet function. Patients with suspected inherited platelet function defects (n = 65, aged 38 ± 19 years, 25% male) were recruited to the GAPP study from UK Comprehensive Care Haemophilia Centers. Participants had a lifelong bleeding history as demonstrated by their elevated ISTH Bleeding Assessment Tool score (median, 10; interquartile range, 7-15; 95th percentile in healthy volunteers was 4).20,21
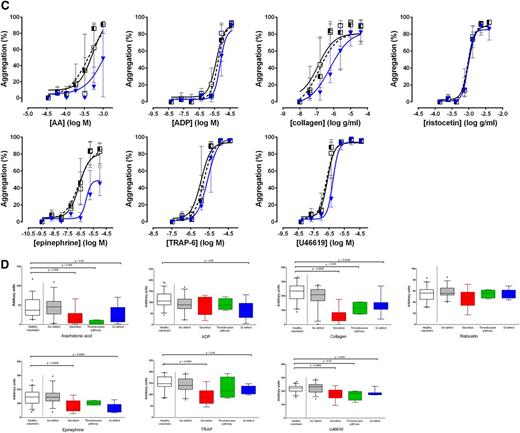
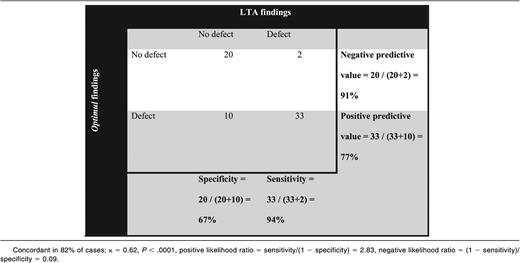
A platelet defect was found in 54% of participants with bleeding symptoms on LTA and in 66% with the Optimul assay (Table 1). The participants’ bleeding history was not predictive of a platelet function defect identified by either LTA or the Optimul assay (Figure 5).21 The assays gave concordant results in 82% of cases (κ = 0.62, P < .0001), suggesting substantial agreement between assays. A normal platelet function result detected by Optimul testing was particularly predictive (sensitivity, 94%; negative predictive value, 91%) of a normal result by reference standard LTA testing. Strikingly, the dose-response curves of patients with bleeding symptoms but in whom no platelet defect was found by lumi-aggregometry were superimposable on the dose-response curves obtained from healthy volunteers (Figure 6), further supporting the high negative predictive value of this assay. This was in line with ROC curve analyses with AUCs not significantly different from 0.5 (supplemental Figure 4A). By contrast, an abnormal platelet function test was detected by Optimul testing in 10 of 65 subjects with suspected platelet function disorders who displayed normal LTA test results. Of the 10 patients with discordant results on Optimul, 7 had impaired collagen responses either in isolation or associated with impairments in other pathways; 1 had a Gi-type presentation; 1 had isolated impairment in AA responses; and 1 had isolated impairment in TRAP responses. The molecular significance of this is unknown, in view of the discrepant results with gold standard lumi-aggregometry and the positive bleeding history suggestive of a platelet defect. Accordingly, Optimul testing showed lower specificity (67%) and positive predictive value (77%) for platelet function disorders compared with the reference standard LTA test. It is not possible to distinguish in this analysis whether this finding indicates a true reduction in Optimul test specificity or whether the Optimul test offers greater sensitivity for the detection of platelet function disorders than LTA.
Distribution of platelet defects in patients with bleeding symptoms suggestive of a platelet defect
Association between presence of a platelet function defect and the ISTH bleeding assessment tool score. Ninety-fifth percentile (score of 4) calculated from healthy volunteers and represented by horizontal dotted line. The line represents the median and the whiskers represent the interquartile range. (A) Presence and absence of a platelet defect defined by lumi-aggregometry. (B) Presence and absence of a platelet defect defined by the Optimul assay.
Association between presence of a platelet function defect and the ISTH bleeding assessment tool score. Ninety-fifth percentile (score of 4) calculated from healthy volunteers and represented by horizontal dotted line. The line represents the median and the whiskers represent the interquartile range. (A) Presence and absence of a platelet defect defined by lumi-aggregometry. (B) Presence and absence of a platelet defect defined by the Optimul assay.
Pattern of platelet responses obtained with Optimul. (A) Dose-response curves presented as median and interquartile range. Open squares represent healthy volunteers, the striped squares represent patients with bleeding symptoms in whom no defect was found on LTA, and red lozenges represent patients in whom a secretion defect was found on LTA. (B) Dose-response curves presented as median and interquartile range. Open squares represent healthy volunteers, the striped squares represent patients with bleeding symptoms in whom no defect was found on LTA, and green circles represent patients in whom a thromboxane pathway defect was found on LTA. (C) Dose-response curves presented as median and interquartile range. Open squares represent healthy volunteers, the striped squares represent patients with bleeding symptoms in whom no defect was found on LTA, and blue triangles represent patients in whom a Gi-type defect was found on LTA. (D) Summary of finding presented as AUC for each of the types of platelet phenotypes defined by LTA. Data presented as median and interquartile range; the whiskers represent the 5th and 95th percentiles.
Pattern of platelet responses obtained with Optimul. (A) Dose-response curves presented as median and interquartile range. Open squares represent healthy volunteers, the striped squares represent patients with bleeding symptoms in whom no defect was found on LTA, and red lozenges represent patients in whom a secretion defect was found on LTA. (B) Dose-response curves presented as median and interquartile range. Open squares represent healthy volunteers, the striped squares represent patients with bleeding symptoms in whom no defect was found on LTA, and green circles represent patients in whom a thromboxane pathway defect was found on LTA. (C) Dose-response curves presented as median and interquartile range. Open squares represent healthy volunteers, the striped squares represent patients with bleeding symptoms in whom no defect was found on LTA, and blue triangles represent patients in whom a Gi-type defect was found on LTA. (D) Summary of finding presented as AUC for each of the types of platelet phenotypes defined by LTA. Data presented as median and interquartile range; the whiskers represent the 5th and 95th percentiles.
Patterns of response in patients with platelet defects
The patients in whom a platelet defect was found by LTA were further subdivided into categories, as recently described by our group.16 The pattern of platelet responses obtained with the Optimul assay for each type of defect based on lumi-aggregometry is shown in Figure 6. ROC curve analyses are presented in supplemental Figure 4B-D.
A secretion defect (defined on lumi-aggregometry as dense granule secretion <5th percentile of normal response to 100 µM TRAP peptide, n = 14 [22%]) was apparent on the Optimul assay as a rightward shift in dose-response in response to most agonists (Figure 6A), except ADP (which adds excess ADP and is relatively insensitive to secretion defects), and ristocetin (which relies on passive agglutination of platelets and does not require platelet activation). It follows that the AUC for all agonists, with the exception of ADP and ristocetin, was significantly lower in patients with secretion defects (Figure 6D and supplemental Figure 4B).
A defect in the thromboxane pathway (defined as either impaired thromboxane generation or thromboxane receptor activity, n = 4 [6%]) appears as a lack of response to AA, accompanied by a significant shift in response to collagen, which strongly relies on thromboxane generation as a secondary mediator (Figure 6B). The thromboxane mimetic (U46619) response can be impaired if there is a defect in the thromboxane receptor, but preserved if there is a defect in the COX enzyme leading to abnormal thromboxane generation. Accordingly, the AUC for these agonists were significantly reduced in comparison with healthy volunteers or patients without a platelet defect (Figure 6D and supplemental Figure 4C).
Finally, a Gi-type defect (defined as a defect in aggregation and secretion to the 2 Gi-coupled receptor agonists ADP and epinephrine, n = 12 [18%]) was the most heterogeneous presentation on Optimul (Figure 6C). Whereas the dose-response curve to epinephrine was systematically impaired, the level of inhibition of the other agonists was significantly more variable with minor rightward shifts in dose-response curves, which nonetheless translated into significant differences on AUC (Figure 6D and supplemental Figure 4D).
Because most platelet defects presented as impairments in AA-induced aggregation, further investigation was undertaken into TxA2 generation in a subset of patients with bleeding symptoms of unknown etiology (n = 35; n = 17 with abnormal AA response on Optimul). Interestingly, although in some participants TxB2 concentration suggested impaired COX activity, in most participants TxB2 concentration fell within ranges compatible with normal COX function (Figure 3), thus shifting the interpretation toward a defect in dense granule/ADP-dependent amplification pathways.
Phenotype-genotype associations
To determine whether the Optimul assay could detect known platelet defects in the main platelet activation pathways, analysis was undertaken in patients with platelet function defects in whom specific mutations had been identified within the GAPP study.
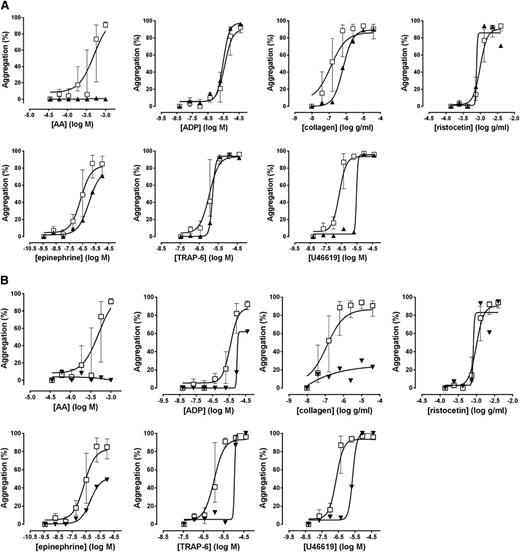
Lumi-aggregometry suggestive of a defect in the thromboxane receptor had led to genotyping of the TBXA2R gene in a participant with a severe bleeding history. A novel heterozygous mutation was found translating into an asparagine to serine change at position 42. This mutation prevents agonist activation of the receptor as a result of poor expression at the cell surface membrane.22 The participant’s Optimul results are shown in Figure 7A and confirmed a marked impairment of AA-induced aggregation with a profound rightward shift in response to the thromboxane mimetic U46619, whereas responses to other agonists were unaltered. Measurement of TxB2 on the highest concentration of the AA-induced aggregation on the Optimul plate demonstrated normal TxA2 generation, despite absence of an aggregation response to AA (40.7 ng/L × 108 platelets; normal range, 19.1-157.6 ng/L × 108 platelets), confirming the defect was at the level of the receptor and not in the enzymatic conversion of AA into TxA2.
Platelet responses obtained with Optimul in patients with known mutations. (A) Patient with a mutation in the TP receptor. (B) Patient with a mutation in the P2Y12 receptor. Dose-response curves presented as median and interquartile range. Open squares represent healthy volunteers, and the black triangles represent the participant with a thromboxane receptor mutation in A and a P2Y12 receptor mutation in B.
Platelet responses obtained with Optimul in patients with known mutations. (A) Patient with a mutation in the TP receptor. (B) Patient with a mutation in the P2Y12 receptor. Dose-response curves presented as median and interquartile range. Open squares represent healthy volunteers, and the black triangles represent the participant with a thromboxane receptor mutation in A and a P2Y12 receptor mutation in B.
Lumi-aggregometry suggestive of a defect in the ADP P2Y12 receptor had led to the identification of a novel heterozygous mutation predicting an arginine to histidine change at position 122. This mutation maps to the DRY motif, a highly conserved region in G-coupled receptors, which was shown in expression studies to inhibit platelet activation by ADP.23,24 Representative Optimul traces from this family are shown in Figure 7B. Impairment in all platelet pathways (except for passive agglutination onto ristocetin) was consistent with a platelet activation defect and was seen in all family members. Further investigation into TxA2 generation confirmed normal COX activity in all family members and reinforced the likelihood that the defect was due to lack of amplification by ADP, with reduced ADP responses suggestive of a defect in ADP receptors.
Discussion
The main findings of this study are as follows: (1) the Optimul assay was robust and reproducible, was completed in a much shorter time after preparation of PRP (10 minutes vs 2 hours) and required significantly less blood than traditional LTA testing (6 vs 50 mL); (2) both assays detected platelet inhibition induced by commonly used antiplatelet agents in samples from healthy volunteers treated with antiplatelets in vitro; (3) both assays detected platelet defects in patients presenting with bleeding symptoms suggestive of a platelet function disorder; (4) Optimul offers high sensitivity for platelet function disorders identified by the reference standard of LTA testing; and (5) Optimul showed lower specificity for platelet function disorders defined by LTA. However, the greater sensitivity of Optimul to platelet inhibition by antiplatelet drugs may suggest that this reflects an inability of LTA to identify some subtle platelet function disorders. The higher sensitivity of the Optimul assay to platelet inhibition and the more global portrait of platelet reactivity to a wider range of agonists and agonist concentrations make it particularly appealing for investigation of platelet phenotypes associated with the complex process of bleeding.
Utility of platelet function testing in mild bleeding disorders
Inherited platelet function disorders are a heterogeneous group of defects, which include defects in platelet receptor function, enzymatic activity, trafficking and granule secretion, and procoagulant activity.1,25 Despite this variety in the molecular basis of the disorder, patients present with a common mucocutaneous bleeding phenotype, which is difficult to distinguish from other more common mild bleeding disorders, for example von Willebrand’s disease.1,25,26 The situation is further complicated by the fact that mucocutaneous bleeding symptoms are reported in a significant proportion of the healthy population, leading to difficulties in determining what level of bleeding is to be considered pathological.27 Laboratory investigation of individuals with bleeding symptoms has thus far suffered from the absence of a test that could monitor a whole spectrum of platelet responses and require a small volume of blood and little technical expertise.4,26 As a consequence, inherited platelet disorders remain underdiagnosed, and under-researched.
We and others have previously shown LTA combined with ATP secretion assessment to be a valuable diagnostic tool.16,28-30 Furthermore, the latest guidelines recommend the use of lumi-aggregometry in the aid to diagnosing inherited platelet defects.26 However, there are wide variations between laboratories using lumi-aggregometry for platelet function testing, which results in lack of standardization.31-33 Moreover, lumi-aggregometry requires specific expertise, both in its performance and its interpretation; expensive machinery; a large volume of blood; and is time and labor intensive.4,30 The latest expert consensus document outlines a number of preanalytical, methodological, and interpretation variables that influence platelet aggregation results.15 Extensive expertise is required in the interpretation of aggregation traces, which should include evaluation of shape change, length of lag phase, slope of aggregation, amplitude of aggregation, deaggregation, and visual examination of each individual trace, making the exercise dependent on the experience of the observer.15 Differences in performance of LTA and interpretation of tracings result in even the best diagnostic laboratories worldwide finding the application of lumi-aggregometry to large groups of patients challenging.31-33 In an effort to standardize and streamline the process, we previously suggested a rationalized and concise panel of agonists to be used with lumi-aggregometry and showed this to be clinically equivalent to using an extensive aggregation panel.16 Nonetheless, the requirement for blood volume and time remains a challenge, especially in circumstances where a large blood volume is difficult to obtain, eg, in children, or in a high-throughput setting such as screening for defects in all-comers with bleeding symptoms. Thus, a high-throughput assay that could monitor a whole spectrum of platelet responses and would require a small volume of blood and little technical expertise would be clinically useful.
Because the Optimul assay is based on light absorbance through a stirred PRP sample, a methodology similar to that used in traditional LTA, we sought to directly compare the methodologies in their efficacy to measure platelet function. The differences in sensitivity to platelet inhibition by these 2 assays serves to stress that despite similarities in the way platelet aggregation is measured between conventional LTA and Optimul, the dynamic forces involved due to either the smaller volumes of PRP used or the different shaking method are sufficient to modify platelet behavior. It is also important to note that the Optimul assay is an end point assay; thus, the increase in throughput is at the expense of the kinetic information available with LTA. Whereas aggregation at 5 minutes incorporates elements of lag phase, maximal amplitude, and deaggregation, visual inspection of the time-dependent nature of aggregation with shape change and primary and secondary wave of aggregation is not available with the Optimul assay.
Potential application of the Optimul assay for platelet function screening
There is increasing demand on physicians for complete information, which partly stems from the desire of patients to avoid unnecessary and predictable complications and to better characterize and understand their symptoms.34 In the context of inherited bleeding disorders, availability of drugs and devices that protect at-risk patients from bleeding complications (eg, desmopressin or antifibrinolytics) renders the diagnosis of a bleeding diathesis clinically relevant.35,36 The Optimul assay could be used as an initial screening assay in patients presenting with bleeding symptoms to direct further investigation, and reduce the time, blood, and labor intensity compared with standard lumi-aggregometry practices. Notwithstanding, the majority of the participants included within GAPP do not present with striking laboratory phenotypes that map to a single platelet activation pathway.1 This finding thus opens up the field of whole-exome genotyping, with the aim of discovering novel mutations to account for the participants’ bleeding and platelet phenotypes. In this setting, the negative discriminative power is crucial to select only those participants with a strong indication for a platelet defect. The Optimul assay used in this fashion could allow high-throughput screening.
From a clinical perspective, the treatment of patients with a platelet defect does not rely on the underlying molecular mechanisms, and thus the demonstration of a clear platelet defect is more clinically valuable than the demonstration of a defect in a specific platelet pathway.34 The application of a high-throughput highly sensitive assay could thus allow for more widespread use of platelet function testing to aid in diagnosis of inherited platelet defects. The ease of use, speed, small blood requirement, and no need for specialized equipment for the performance of the Optimul assay render it particularly appealing, especially outside of large tertiary centers, and could help alleviate the burden of platelet function testing in patients presenting with bleeding. Its use in this context, however, requires large clinical studies to be carried out.
Conclusion
This study demonstrates that the Optimul assay, which exploits the advantages of the 96-well plate format, is useful in characterizing platelet function in healthy volunteers and in patients presenting with bleeding suggestive of an inherited platelet function defect. It offers distinct advantages in terms of being faster, requiring less blood, and allowing AUCs to be determined, but the tradeoffs include greater complexity until a commercial equivalent can be devised and the loss of kinetic information. Applying this assay to the 65 participants in this study as a screening assay could have avoided detailed phenotyping with lumi-aggregometry in 22 participants (34%) and would have correctly identified 33 participants (51%) as having a platelet defect. This benefit would have occurred at the cost of 2 missed diagnoses of platelet defects and 10 extra diagnoses that were later not substantiated by lumi-aggregometry. In a clinical setting, the high negative predictive value of the Optimul assay makes it particularly appealing as a screening assay in nonspecialized centers. Indeed, its use could prevent extra platelet function testing in up to a third of patients with excessive bleeding and could select the remaining patients to be referred on for more detailed phenotyping in a specialized center.
Although this assay is not quite yet a “lab on a chip,” it provides a multifaceted portrait of platelet function that is frequently lacking in current platelet function assays. Future studies are needed to explore whether the Optimul assay used as an initial screening tool, in conjunction with a rationalized LTA panel, may be useful in patients with a clinical history of mild bleeding to identify hidden genetic hemostatic defects, which often go unnoticed until a challenge, such as surgery or addition of an antiplatelet agent, unveils the underlying defect.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The GAPP programme is chaired by S.P.W. and has principal investigators in Bristol (Andrew Mumford and Stuart Mundell), London (Paul Gissen), and Sheffield (Martina Daly).
This work was supported by the British Heart Foundation (BHF; RG/09/007/27917 and PG/10/36/02) and the Wellcome Trust (093994). S.P.W. holds a BHF chair (CH/03/003). M.L. is supported by the Canadian Institute of Health Research (MFE-107592) and the BHF (PG/11/31/28835). G.C.L. and N.S.K. are supported by the Wellcome Trust (093994 and 085255/Z/09/Z). M.V.C. is supported by the William Harvey Research Foundation. M.H.L. is supported by the BHF (PG/11/75/29105).
Authorship
Contribution: M.L. designed the research, performed the assays and collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; G.C.L. and N.S.K. collected data, analyzed and interpreted data, and revised the manuscript; M.V.C., M.H.L., N.V.M., D.B., S.P.N., V.C.L., M.L.J., S.J.M., M.E.D., and A.D.M. analyzed and interpreted data and revised the manuscript; and T.D.W. and S.P.W. designed the research, analyzed and interpreted data, and revised the manuscript. A complete list of the members of the UK Genotyping and Phenotyping of Platelets Study Group appears in the Appendix.
Conflict-of-interest disclosure: M.L. has received speaker honoraria from Eli Lilly and has served as a consultant for Multiplate (Roche). The remaining authors declare no competing financial interests.
Correspondence: Marie Lordkipanidzé, Research Center, Montreal Heart Institute, 5000 rue Bélanger, Montréal, QC H1T 1C8, Canada; e-mail: marie.lordkipanidze@umontreal.ca.
Appendix
The members of the UK Genotyping and Phenotyping of Platelets Study Group are: Steve P. Watson (University of Birmingham); Andrew D. Mumford and Stuart J. Mundell (University of Bristol); Paul Gissen (University College London); Martina E. Daly (University of Sheffield); Will Lester and Justin Clark (Birmingham Women's Hospital); Mike Williams, Jayashree Motwani, Dianne Marshall, Priscilla Nyatanga, Pat Mann, and Julie Kirwan (Birmingham Children's Hospital); Jonathan Wilde, Tracey Dunkley, and April Greenway (University Hospital Birmingham); Michael Makris (Sheffield Haemophilia and Thrombosis Centre, Royal Hallamshire Hospital); Sue Pavord and Rashesh Dattani (University Hospitals Leicester); Gerry Dolan Charlotte Grimley, Simone Stokley, Emma Astwood, Cherry Chang, Merri Foros, and Linda Trower (Nottingham University Hospitals); Jecko Thachil (previously Paula Bolton-Maggs), Charlie Hay, Gill Pike, Andrew Will, John Grainger, Matt Foulkes, and Mona Fareh (Central Manchester National Health Service [NHS] Foundation Trust); Kate Talks, Tina Biss, Patrick Kesteven, John Hanley, Julie Vowles, Lesley Basey, and Michelle Barnes (Newcastle upon Tyne Hospitals NHS Trust); Peter Collins, Rachel Rayment, Raza Alikhan, Ana Guerrero Rebecca Morris, and Dianne Mansell (Cardiff and Vale University Local Health Board); Cheng Hock Toh and Vanessa Martlew (Royal Liverpool University Hospitals); Elaine Murphy and Robin Lachmann (University College London Hospitals NHS Trust); Peter Rose, Oliver Chapman, Anand Lokare, Kathryn Marshall, and Naseem Khan (University Hospitals Coventry and Warwickshire); David Keeling and Paul Giangrande (Oxford Radcliffe Hospitals NHS Trust); and Steve Austin, David Bevan and Jayanthi Alamelu (Guys' and St. Thomas' NHS Foundation Trust).