Key Points
An injection of heat-killed Brucella abortus in mice causes prolonged anemia with features similar to human anemia of inflammation.
Ablation of hepcidin ameliorates anemia of inflammation in this model and allows faster recovery.
Abstract
Anemia is a common complication of infections and inflammatory diseases, but the few mouse models of this condition are not well characterized. We analyzed in detail the pathogenesis of anemia induced by an injection of heat-killed Brucella abortus and examined the contribution of hepcidin by comparing wild-type (WT) to iron-depleted hepcidin-1 knockout (Hamp-KO) mice. B abortus–treated WT mice developed severe anemia with a hemoglobin nadir at 14 days and partial recovery by 28 days. After an early increase in inflammatory markers and hepcidin, WT mice manifested hypoferremia, despite iron accumulation in the liver. Erythropoiesis was suppressed between days 1 and 7, and erythrocyte destruction was increased as evidenced by schistocytes on blood smears and shortened red blood cell lifespan. Erythropoietic recovery began after 14 days but was iron restricted. In B abortus–treated Hamp-KO compared with WT mice, anemia was milder, not iron restricted, and had a faster recovery. Similarly to severe human anemia of inflammation, the B abortus model shows multifactorial pathogenesis of inflammatory anemia including iron restriction from increased hepcidin, transient suppression of erythropoiesis, and shortened erythrocyte lifespan. Ablation of hepcidin relieves iron restriction and improves the anemia.
Introduction
Anemia of inflammation (AI) is a feature of a wide spectrum of inflammatory disorders, including connective tissue disease, infections, certain malignancies, and chronic kidney disease.1 AI is typically a normocytic normochromic anemia with a shortened erythrocyte lifespan and suppressed erythropoiesis, despite adequate levels of circulating erythropoietin.2 Perhaps the most consistent feature of AI is a derangement of systemic iron homeostasis characterized by hypoferremia with intact iron stores1 and decreased availability of iron for erythrocyte production.
Hepcidin, a 25-amino acid peptide hormone produced primarily by hepatocytes,3 is the principal regulator of iron homeostasis in health and during inflammation.4 Excessive production of this hormone causes iron sequestration in macrophages and hypoferremia, as was shown in transgenic mice with hepcidin overexpression5 and in the human genetic syndrome of hepcidin excess, iron-refractory iron-deficiency anemia due to mutations in matriptase-2/TMPRSS6.6 Hepcidin acts by binding to ferroportin, the sole known cellular iron exporter, displayed on the surface of macrophages, hepatocytes, and the basolateral membranes of enterocytes. Hepcidin binding to ferroportin causes ferroportin endocytosis and degradation.7 During inflammation or infection, hepcidin is strongly induced, largely by interleukin 6 (IL-6)8 via the Janus kinase–signal transducer and activator of transcription pathway.9-11 The extent to which increased production of hepcidin contributes to AI has not been directly tested by genetic ablation of hepcidin, in part because of the lack of robust mouse models of AI.
Multiple mouse models of inflammation have been developed to facilitate the testing of interventions in AI, but these models are limited by poor reproducibility and very mild anemia. Turpentine,12,13 zymosan/lipopolysaccharide,14 Staphylococcus epidermidis,15 and complete Freund’s adjuvant15 treatments all cause AI in mice but with a mild anemia (hemoglobin [Hgb] ≥11 g/dL). Lipopolysaccharide injections alone have been shown to cause an acute inflammatory response with hepcidin elevation, but the hematologic effects have not been fully elucidated.16 Cecal ligation and puncture is another potential method for studying AI, but its technical difficulty, variable outcomes, and associated morbidity make it cumbersome for routine use.
In response to the need for a robust mouse model of AI, we adapted and characterized a model of AI previously described by Sasu et al,17 generated with a single intraperitoneal injection of heat-killed Brucella abortus. After a comprehensive examination of hematologic, iron, and inflammatory parameters of this model, we investigated the extent of hepcidin involvement in B abortus–induced AI by comparing wild-type (WT) C57BL/6 mice to hepcidin-1−/− mice on the same strain background.
Methods
Animal model of AI
All animal studies were approved by the Animal Research Committee at University of California, Los Angeles (UCLA). Only male mice were used in the study to avoid the effects of gender-related differences in iron parameters and hepcidin.18 C57BL/6J mice were obtained from Charles River Laboratories (Wilmington, MA) or The Jackson Laboratories (Bar Harbor, ME). WT mice were maintained on a standard chow (∼270 ppm iron; Harlan Teklad, Indianopolis, IN) until 6 weeks of age, and they were then switched to an adequate iron diet (50 ppm iron; Harlan Teklad) for 2 weeks before injection of B abortus or saline. The same iron-adequate diet was used through the remainder of the experiment. The dietary conditioning was applied because high iron content of standard chow maximally stimulates hepcidin expression and renders it unresponsive to inflammatory stimuli8 and because dietary iron absorption in humans accounts for ∼5% to 10% of the daily iron fluxes but as much as ∼50% in mice fed standard chow.19 High dietary iron absorption in mice may obscure the contribution of iron recycling by macrophages20 and leads to progressive iron loading. Reducing the dietary iron content of mouse chow was designed to model iron fluxes of human homeostasis.
To induce AI, animals were injected intraperitoneally with 5 × 108 particles per mouse of heat-killed B abortus (strain 1119-3; US Department of Agriculture, Animal and Plant Health Inspection Service, National Veterinary Services Laboratories) as previously described.17 Control mice were injected intreperitoneally with normal saline. Mice were euthanized over a time course (0-28 days), and blood, liver, spleen, pancreas, and kidney were collected at necropsy. For each time point, 8 saline and 8 B abortus–injected mice came from the same cohort and were processed simultaneously to minimize cohort-to-cohort variability, yielding 4 to 8 evaluable samples per group per time point.
For the study of the role of hepcidin in AI, we used male hepcidin-1 knockout mice (Hamp1−/−or Hamp-KO). Hamp-KO mice were originally provided to our laboratory by Dr Sophie Vaulont21 and were backcrossed onto the C57BL/6 background as previously described22 using marker-assisted accelerated backcrossing. For this study, Hamp-KO mice underwent dietary iron conditioning to prevent the development of iron overload and maintain iron levels comparable to those of WT mice. At weaning (3-4 weeks of age), Hamp-KO mice were placed on a low-iron diet (4 ppm) for 2 weeks followed by a 20 ppm iron diet for 2 weeks. This regimen allows for iron depletion without development of anemia. At 7 to 8 weeks of age, the mice were injected with B abortus or saline and switched to an adequate iron diet (50 ppm) to match the experimental conditions used for WT mice. Hamp-KO mice (3-8 evaluable per group and time point) were analyzed before and 7, 14, 21, and 28 days after B abortus or saline injection.
Measurement of iron parameters and erythropoietin
Serum iron and liver nonheme iron concentrations were determined by a colorimetric assay for iron quantification (Sekisui Diagnostics, Lexington, MA) as previously described.19 Serum erythropoietin was measured by a solid-phase enzyme-linked immunosorbent assay according to the manufacturer’s instructions (R&D, Minneapolis, MN).
Hematological studies
Complete blood counts were obtained with a HemaVet blood analyzer (Drew Scientific, Waterbury, CT). To assess iron-restricted erythropoiesis, zinc protoporphyrin (ZPP) was measured using a hematofluorometer (AVIV, Lakewood, NJ).
Reticulocytes were counted by flow cytometry. Blood (5 µL) was added to 1 mL of thiazole orange in phosphate-buffered saline with 0.1% sodium azide (PBS-azide; BD Bioscience; San Jose, CA) and incubated at room temperature for 1 to 3 hours. As an unstained control, blood was added to PBS-azide without thiazole orange. The percentage of red fluorescent reticulocytes (Retic %) was measured by flow cytometry at the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health Awards CA-16042 and AI-28697, and by the Jonsson Comprehensive Cancer Center , the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA. Unstained controls were used to establish a gate to exclude background fluorescence. The results are expressed as the reticulocyte product index = Retic % × Hgb/14.46 g/dL, where 14.46 g/dL is a mean baseline Hgb level of healthy WT mice.
In vivo erythrocyte biotinylation
WT mice were injected intraperitoneally with saline or B abortus. On days 5 and 6, the mice were administered n-hydroxysuccinimide ester-(polyethylene glycol)4-biotin (NHS-PEG4-Biotin, 500 μg; Pierce, Rockford, IL) in PBS by retro-orbital injection. A total of 5 injections were given to maximize biotinylation efficiency. Over the subsequent 2 weeks, periodic retro-orbital bleeds (25-50 μL) were obtained to quantify the fraction of biotinylated erythrocytes (timeline indicated in supplemental Figure 1 on the Blood Web site). Each sample was divided into 2 aliquots: one to be labeled with anti–TER-119 (unstained) and the other with anti–TER-119 and streptavidin (stained). The labeling was performed as follows: 5 μL whole blood was diluted to 500 μL with Hank’s balanced salt solution (Invitrogen, Carlsbad, CA) and 1% bovine serum albumin (Rockland, Gilbertsville, PA), and 2.5 μL rat anti-mouse TER-119-phycoerythrin added (BD Pharmingen, San Jose, CA). The stained group was also treated with 0.5 μL streptavidin-Alexa 488 (Molecular Probes, Eugene, OR). After incubation at room temperature for 1 hour, the samples were centrifuged and resuspended with Hank’s balanced salt solution/bovine serum albumin. Flow cytometry was used to determine the percentage biotinylated erythrocytes (% stained – % unstained).
Serum hepcidin measurement
Mouse serum hepcidin-1 was measured by a sandwich enzyme-linked immunosorbent assay23 (courtesy of B. Sasu and K. Cooke, Amgen, Thousand Oaks, CA). The assay was physiologically validated in our laboratory as described in the supplemental data.
RNA isolation and real-time quantitative polymerase chain reaction
Total RNA was isolated from liver and analyzed by real-time reverse transcriptase-polymerase chain reaction as described previously.24 Primers are listed in supplemental Table 1.
Histocytopathology
Peripheral blood smears were performed using 10 μL whole blood at the time of necropsy, and prepared with Wright-Giemsa stain (Fisher, Hampton, NH). Formalin-fixed, paraffin-embedded liver and kidney tissues were stained with hematoxylin and eosin, periodic acid-Schiff, or Masson's trichrome stain at the UCLA Translational Pathology Core Laboratory. Immunoperoxidase staining with 1 μg/mL rat anti-mouse monoclonal antibody to the macrophage marker F4/80 (AbD Serotec, Kidlington, United Kingdom) was performed on deparaffinized sections after unmasking for 3 minutes with 20 μg/mL proteinase K. Sections were incubated overnight with the anti-F4/80 Mab solution, developed, and counterstained with hematoxylin, all using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
Statistics
SigmaStat 11 was used for all statistical analyses (Systat Software, Point Richmond, CA). Normally distributed data were compared using the Student t test. Measurements that were not normally distributed were compared by the nonparametric Mann-Whitney U test. P < .05 was considered statistically significant, except for multiple comparisons where the Bonferroni correction was applied.
Results
B abortus–treated WT mice develop severe anemia with iron restriction
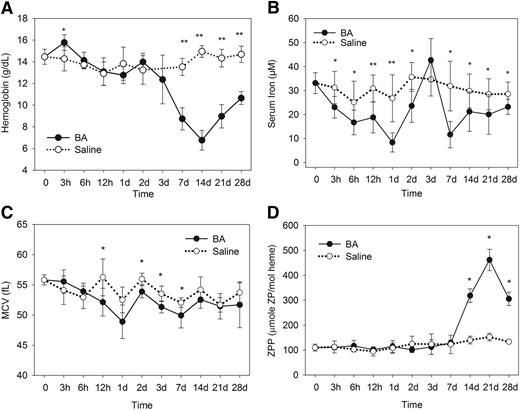
A single treatment with the inflammatory agent B abortus caused a dramatic hemoglobin decrease by 7 days with a nadir at 14 days (Figure 1A; mean B abortus Hgb 6.8 g/dL vs saline Hgb 15.0 g/dL; P < .001 by Mann-Whitney U test). Afterward, there was a gradual but incomplete hemoglobin recovery by day 28 (B abortus Hgb 10.7 g/dL vs saline Hgb 14.7 g/dL; P < .001 by Student t test).
The B abortus–injected mice develop anemia and iron restriction. Compared with saline-injected mice, the B abortus–injected mice develop (A) a significantly lower hemoglobin by 7 days, with a nadir at 14 days and partial recovery by 28 days; (B) decreased serum iron concentrations through most time points, beginning at 3 hours, except for the transient underutilization of iron around day 3 during erythropoiesis suppression; (C) decreased MCVs within the first week; and (D) elevated ZPP levels between 14 and 28 days during the time of recovery from anemia. Saline and B abortus groups each included 4 to 8 evaluable male mice per time point. Means ± standard deviation (SD) are shown; *P < .05 and **P < .001 by Student t test or Mann-Whitney U test.
The B abortus–injected mice develop anemia and iron restriction. Compared with saline-injected mice, the B abortus–injected mice develop (A) a significantly lower hemoglobin by 7 days, with a nadir at 14 days and partial recovery by 28 days; (B) decreased serum iron concentrations through most time points, beginning at 3 hours, except for the transient underutilization of iron around day 3 during erythropoiesis suppression; (C) decreased MCVs within the first week; and (D) elevated ZPP levels between 14 and 28 days during the time of recovery from anemia. Saline and B abortus groups each included 4 to 8 evaluable male mice per time point. Means ± standard deviation (SD) are shown; *P < .05 and **P < .001 by Student t test or Mann-Whitney U test.
B abortus–injected mice rapidly developed a prolonged hypoferremia compared with saline-treated mice (Figure 1B). There was a transient increase in serum iron at 3 days, which was attributed to transient erythroid suppression and underutilization of circulating iron, as documented by subsequent data. However, all other time points showed a significant decrease in serum iron (P < .05 by Student t test). Additionally, within the first week, the mean corpuscular volume (MCV) was lower in the B abortus–treated mice, consistent with iron-restricted anemia25 (Figure 1C). Later MCV measurements (14-28 days) in the B abortus group were not significantly different from the control group likely because of the increased number of larger stress reticulocytes in B abortus mice.
Erythrocyte ZPP levels were measured to further assess iron-restricted erythropoiesis (Figure 1D). When insufficient iron is available for erythropoiesis, increased amounts of zinc are incorporated into the protoporphyrin ring.26 The B abortus–treated mice had comparable ZPP levels to untreated mice until a dramatic increase by 14 days (mean B abortus ZPP 319 μM/mole heme vs saline ZPP 140 μM/mole heme; P < .001 by Student t test), with an eventual peak at 21 days (B abortus ZPP 462 μM/mole heme vs saline ZPP 152 μM/mole heme; P < .001 by Mann-Whitney U test) and a partial recovery of B abortus ZPP by 28 days. As will be seen subsequently, the ZPP increase coincides with accelerated erythropoiesis during recovery from anemia.
B abortus–treated WT mice develop acute and chronic inflammation, with an early rise in hepcidin
We assessed the acute phase response to B abortus by measuring serum amyloid A1 (SAA1) messenger RNA (mRNA), which like hepcidin, is regulated by IL-6.27 B abortus–treated mice developed an early-onset and persistent SAA1 mRNA elevation starting by 3 hours and lasting through 14 days (Figure 2A). In parallel with the increase in SAA1, hepcidin mRNA levels (Figure 2B) peaked at 6 hours with a greater than sixfold increase over their saline counterparts (P < .05 by Student t test), and serum hepcidin protein levels (Figure 2C; see the supplemental data for assay validation) were increased by greater than threefold at 6 hours (P < .001 by Student t test). Subsequently, hepcidin mRNA levels fell rapidly to levels similar to those of the saline-treated mice, perhaps responding to the developing hypoferremia (Figure 1B). However, normal hepcidin levels at 7 days are probably inappropriately high28 considering the profound anemia and very high Epo production in B abortus mice (Figure 3) at that time point. By 14 days, B abortus–treated mice had significantly decreased hepcidin mRNA (<10% compared with saline controls; P < .001 by Student t test), presumably secondary to the known suppressive effect of increased erythropoiesis on hepcidin production.28
The B abortus–injected mice develop both acute and chronic inflammation, with an early rise in hepcidin mRNA. Compared with saline-injected mice, the B abortus–injected mice develop (A) increased SAA-1 mRNA by 3 hours, peaking at 1 day, and a gradual return to normal by 21 days; (B) increased hepcidin mRNA, with a peak at 6 hours, a slight but consistent increase through 7 days, followed by significant decrease at 14 and 28 days; (C) increased serum hepcidin protein at 6 hours; (D) increased WBCs by 7 days, with partial recovery by 28 days; and (E) hepatic perivascular infiltration of inflammatory cells by 7 days. (A-B) Means ± standard error, with each B abortus–treated group referenced to contemporaneous saline-treated group. (C-D) Means ± SD, *P < .05 and **P < .001 by Student t test or Mann-Whitney U test.
The B abortus–injected mice develop both acute and chronic inflammation, with an early rise in hepcidin mRNA. Compared with saline-injected mice, the B abortus–injected mice develop (A) increased SAA-1 mRNA by 3 hours, peaking at 1 day, and a gradual return to normal by 21 days; (B) increased hepcidin mRNA, with a peak at 6 hours, a slight but consistent increase through 7 days, followed by significant decrease at 14 and 28 days; (C) increased serum hepcidin protein at 6 hours; (D) increased WBCs by 7 days, with partial recovery by 28 days; and (E) hepatic perivascular infiltration of inflammatory cells by 7 days. (A-B) Means ± standard error, with each B abortus–treated group referenced to contemporaneous saline-treated group. (C-D) Means ± SD, *P < .05 and **P < .001 by Student t test or Mann-Whitney U test.
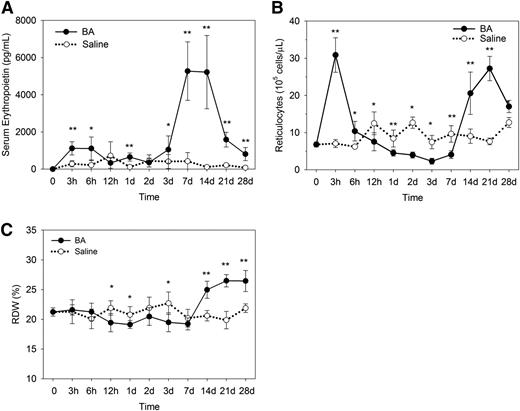
Suppression and recovery of erythropoiesis in B abortus–treated mice. (A) Serum erythropoietin concentrations in B abortus–treated mice increase to a peak by 7 days, with a decline approaching normal values by 28 days. (B) After the initial release of reticulocytes at 3 hours, reticulocytes decrease significantly below those of saline-treated mice from 12 hours to 7 days, followed by reticulocytosis on days 14 to 28. (C) Consistent with decreased reticulocytes in B abortus–treated mice, the red cell distribution width is suppressed at 12 hours to 3 days but increases by 14 days. Means ± SD are shown. *P < .05 and **P < .001 by Student t test or Mann-Whitney U test.
Suppression and recovery of erythropoiesis in B abortus–treated mice. (A) Serum erythropoietin concentrations in B abortus–treated mice increase to a peak by 7 days, with a decline approaching normal values by 28 days. (B) After the initial release of reticulocytes at 3 hours, reticulocytes decrease significantly below those of saline-treated mice from 12 hours to 7 days, followed by reticulocytosis on days 14 to 28. (C) Consistent with decreased reticulocytes in B abortus–treated mice, the red cell distribution width is suppressed at 12 hours to 3 days but increases by 14 days. Means ± SD are shown. *P < .05 and **P < .001 by Student t test or Mann-Whitney U test.
The B abortus–treated mice also developed chronic inflammation. By 7 days, the B abortus–treated mice developed a dramatic leukocytosis (B abortus white blood cell count [WBC] 29.6 thousand [K]/μL vs saline WBC 8.2 K/μL; P < .001 by Student t test), which incompletely resolved by day 28 (B abortus WBC 11.6 K/μL vs saline WBC 6.7 K/μL; P < .05 by Student t test; Figure 2D). Figure 2E demonstrates the hepatic perivascular infiltration of inflammatory cells in B abortus–treated mice at 7 days, which is contemporaneous with the increase in circulating white blood cells. By 14 days, hepatic granulomas were observed containing predominantly F4/80-positive macrophages (supplemental Figure 2A-B).
Suppression and recovery of erythropoietic response in B abortus–treated mice
By 7 days, serum erythropoietin levels were increased in B abortus–treated mice (Figure 3A; >12-fold increase over controls; P < .001 by Student t test), consistent with anemia (Figure 1A). By 21 days, as the anemia began to resolve, serum erythropoietin of the B abortus–treated mice started to fall (less than eightfold increase over controls; P < .001 by Mann-Whitney U test).
Reticulocyte measurements showed initial reticulocytosis at 3 hours after B abortus injection (Figure 3B), but this is likely due to massive release of stored near-mature reticulocytes rather than new production. During the first week, reticulocyte production decreased in B abortus mice (Figure 3B), indicating erythropoietic suppression. By 14 days, corresponding to the time of the highest erythropoietin levels and lowest hepcidin expression, reticulocytosis increased and peaked at 21 days (more than threefold increase over controls; P < .001 by Mann-Whitney U test). Because “stress” reticulocytes produced during stress erythropoiesis are significantly larger than mature erythrocytes, an increased percentage of reticulocytes effectively increased the red cell distribution width between days 14 and 28 (Figure 3C).
Shortened red blood cell lifespan and increased red blood cell hemolysis contribute to B abortus–induced anemia
A red blood cell (RBC) biotinylation assay to compare the RBC lifespans of B abortus–treated and saline control mice (Figure 4A) demonstrated a 2.5-fold shortening of the erythrocyte lifespan in B abortus–treated mice, indicating that increased RBC destruction was contributing to the anemia. Erythrocyte abnormalities in B abortus–treated mice were most prominent at 14 days (Figure 4B), with schistocytes and RBC membrane irregularities indicative of membrane damage. However, the condition was relatively mild, as indicated by schistocytes comprising <1% of erythrocytes even at 14 days (see the next section). For comparison, in a human disorder with prominent microangiopathic hemolysis such as the hemolytic-uremic syndrome, the percentage schistocytes ranged from 2% to 9%.29
B abortus–treated mice have shortened RBC lifespan and evidence of hemolysis. (A) The RBC biotinylation assay indicates a 2.7-fold increase in RBC destruction in B abortus–treated mice (−4%/day vs −1.7%/day). Means ± SD. *P < .05 and **P < .001 by Student t test or Mann-Whitney U test. Overall difference between the 2 sets of measurements is significant at P < .001 by 2-way analysis of variance (Holm-Sidak method). (B) Peripheral blood smears of B abortus–injected mice 2 weeks after showing schistocytes and erythrocyte membrane irregularities, consistent with mild microangiopathic hemolysis. Arrows point to damaged erythrocytes, shown magnified in the right margin.
B abortus–treated mice have shortened RBC lifespan and evidence of hemolysis. (A) The RBC biotinylation assay indicates a 2.7-fold increase in RBC destruction in B abortus–treated mice (−4%/day vs −1.7%/day). Means ± SD. *P < .05 and **P < .001 by Student t test or Mann-Whitney U test. Overall difference between the 2 sets of measurements is significant at P < .001 by 2-way analysis of variance (Holm-Sidak method). (B) Peripheral blood smears of B abortus–injected mice 2 weeks after showing schistocytes and erythrocyte membrane irregularities, consistent with mild microangiopathic hemolysis. Arrows point to damaged erythrocytes, shown magnified in the right margin.
Microscopic examination of hematoxylin and eosin, periodic acid-Schiff, and Masson trichrome stains of liver, kidney, spleen, and pancreas sections obtained at autopsies of B abortus– and saline-treated mice revealed foci of thrombosis in small arteries and veins in the liver and the kidneys (supplemental Figure 2C-D), as well as in peripancreatic adipose tissue (data not shown), indicative of endothelial injury and microthrombosis that could cause microangiopathic hemolysis.
Contribution of hepcidin to B abortus–induced hypoferremia and anemia
We next examined the development of hypoferremia and anemia in B abortus–treated Hamp-KO. Prior to use in this study, Hamp-KO mice were placed for 4 weeks on an iron-restricted diet to prevent the massive iron overload characteristic of Hamp-KO mice, so that they were as similar as possible to WT mice except for the effect of hepcidin during inflammation in the latter. Iron-depleted Hamp-KO mice had similar blood hemoglobin as their WT counterparts (Figure 5A). Like WT mice, B abortus–treated Hamp-KO mice became inflamed, as indicated by increased SAA-1 mRNA levels and leukocytosis (supplemental Figure 3). Like WT mice, Hamp-KO mice showed erythrocyte fragments on blood smears, most prominently at 14 days (0.7 ± 0.5% of erythrocytes in WT, 0.5 ± 0.1% in Hamp-KO, n = 4 mice each group, difference not significant by Student t test). Both B abortus–treated groups (Hamp-KO and WT) reached their hemoglobin nadir at 14 days, but the anemia of the Hamp-KO mice was much milder than in the WT mice (Hamp-KO B abortus Hgb 9.8 g/dL vs WT B abortus Hgb 6.8 g/dL; P < .001 by Mann-Whitney U test). Moreover, the Hamp-KO B abortus–treated mice recovered completely by 28 days, whereas the WT B abortus mice did not (Hamp-KO B abortus Hgb 15.1 g/dL vs WT B abortus Hgb 10.7 g/dL; P < .001 by Student t test).
Hepcidin ablation attenuates B abortus–induced anemia and iron restriction. (A) B abortus–treated Hamp-KO mice (HKO) develop milder anemia than their WT counterparts and completely recover by 28 days. Hamp-KO B abortus vs WT B abortus, day 7 or 21, P < .05; Hamp-KO B abortus vs WT B abortus, day 14 or 28, P < .001, n = 3 to 8 per time point. (B) Unlike their WT counterparts, B abortus–injected Hamp-KO mice increase their serum iron levels. Hamp-KO B abortus vs Hamp-KO saline, day 14, P < .05; WT B abortus vs WT saline, days 7, 14, 21, and 28, P < .05. (C) B abortus–treated WT mice have dramatically increased RBC ZPP, indicating iron-restricted erythropoiesis, compared with the very modest increases of ZPP in Hamp-KO mice. (D) B abortus–treated Hamp-KO mice mobilize iron from liver iron stores, whereas their WT counterparts mildly increase their stores in the face of anemia. Hamp-KO B abortus vs Hamp-KO saline, day 14, P < .001; day 28, P < .05. WT B abortus vs WT saline, day 7 or 14, P < .001; day 21, P < .05. Means ± SD are shown. P value by Student t test or Mann-Whitney U test. WT data shown in A-C are identical to those from Figure 1A-B,D.
Hepcidin ablation attenuates B abortus–induced anemia and iron restriction. (A) B abortus–treated Hamp-KO mice (HKO) develop milder anemia than their WT counterparts and completely recover by 28 days. Hamp-KO B abortus vs WT B abortus, day 7 or 21, P < .05; Hamp-KO B abortus vs WT B abortus, day 14 or 28, P < .001, n = 3 to 8 per time point. (B) Unlike their WT counterparts, B abortus–injected Hamp-KO mice increase their serum iron levels. Hamp-KO B abortus vs Hamp-KO saline, day 14, P < .05; WT B abortus vs WT saline, days 7, 14, 21, and 28, P < .05. (C) B abortus–treated WT mice have dramatically increased RBC ZPP, indicating iron-restricted erythropoiesis, compared with the very modest increases of ZPP in Hamp-KO mice. (D) B abortus–treated Hamp-KO mice mobilize iron from liver iron stores, whereas their WT counterparts mildly increase their stores in the face of anemia. Hamp-KO B abortus vs Hamp-KO saline, day 14, P < .001; day 28, P < .05. WT B abortus vs WT saline, day 7 or 14, P < .001; day 21, P < .05. Means ± SD are shown. P value by Student t test or Mann-Whitney U test. WT data shown in A-C are identical to those from Figure 1A-B,D.
As expected, compared with WT mice, serum iron in Hamp-KO mice remained elevated even on an iron-restricted diet that substantially depleted their iron stores (Figure 5B). Unlike WT mice, the B abortus–treated Hamp-KO mice did not develop hypoferremia, but on the contrary, showed increased serum iron. At 14 days, the B abortus–treated Hamp-KO mice had a nearly twofold increase in serum iron compared with saline-treated Hamp-KO mice (P < .05 by Mann-Whitney U test). Higher serum iron is likely due to increased destruction of RBCs after B abortus treatment and recycling of their iron content. ZPP, an index of iron-restricted erythropoiesis, changed very little in B abortus–treated Hamp-KO mice compared with WT mice (Figure 5C).
Compared with the saline Hamp-KO group, B abortus–treated Hamp-KO mice transiently decreased their liver iron stores at 7 and 14 days, indicating that Hamp-KO mice mobilized iron from stores in response to anemia. In contrast, despite anemia and increased erythropoietic activity, B abortus–treated WT mice had increased liver iron stores compared with their saline counterparts, suggesting that hepcidin prevented efficient mobilization of iron from the liver (Figure 5D).
Interestingly, Hamp-KO mice had significantly decreased survival compared with their WT counterparts (supplemental Figure 4). Thus, although hepcidin ablation was protective against the development of severe anemia and iron restriction, hepcidin deficiency or the associated iron redistribution may have impacted B abortus–induced inflammation or its damaging effects on tissues and organs.
Discussion
AI (also called anemia of chronic disease) is a mild to moderate, usually normocytic anemia seen in the context of infections and systemic inflammatory disorders including rheumatologic disorders, inflammatory bowel diseases, and malignant neoplasms.1 In addition, chronic kidney diseases often manifest inflammation and associated features of AI. A related condition, anemia of critical illness, develops in intensive care unit settings within days.30,31 The pathogenesis of AI is thought to involve a prominent component of inflammation-induced iron restriction, as well as impaired proliferation of erythroid precursors, resistance to erythropoietin, and shortened erythrocyte survival,1,32 and may be further exacerbated by blood loss and iron deficiency. Progress in understanding these and additional mechanisms has been hampered by the lack of a simple and reproducible animal model that can be genetically manipulated.
In this study, we extensively characterized a mouse model of AI induced by heat-killed B abortus. In contrast to other mouse models of AI, where anemia was generally very mild, the B abortus–induced anemia results in a nadir hemoglobin that is <50% of the controls. Remarkably, the B abortus model is technically simple, requiring only a single injection, and it is robust and reproducible at each time point, with small standard deviations for most hematologic, iron, and inflammatory parameters measured. The model has features of both acute and chronic inflammation, as evidenced by the early SAA-1 increase and the delayed leukocytosis and hepatic perivascular infiltration of inflammatory cells.
Clinically, this subacute mouse model of moderately severe AI resembles human AI in severe infections, or in critically ill patients in intensive care units, or in patients with severe exacerbations of systemic autoimmune diseases. It remains to be tested whether lower dosing of heat-killed B abortus with repeated administration would result in a less severe and more chronic model, with steady-state anemia similar to classical anemia of chronic disease.
Our mouse model manifests the key mechanisms implicated in human AI, including hypoferremia and iron-restricted erythropoiesis with preserved iron stores, shortened erythrocyte lifespan, and depressed erythropoiesis. Hypoferremia developed rapidly, probably as a consequence of acute increase in hepcidin mRNA and plasma levels driven by inflammation after B abortus injection. Although following the initial rise hepcidin fell to control levels during the first week, this may not have been sufficiently low in the face of the developing anemia, further contributing to iron restriction. Elevated liver iron in B abortus–treated WT mice compared with their saline counterparts further supports the role of hepcidin in preventing effective iron mobilization for the recovery from anemia.
The shortened erythrocyte lifespan in the B abortus mouse model may be a consequence of macrophage activation by cytokines32,33 and/or microangiopathic hemolysis. In support of the latter mechanism, arteriolar and venous microthrombosis was observed in multiple tissues, and fragmented red cells were apparent on blood smears. However, we did not detect the characteristic deposition of fibrin in renal glomerular capillaries, and schistocytes were relatively rare, suggesting that the condition was not severe. Increased erythrophagocytosis by inflammation-activated macrophages was documented in the companion article34 and may well be the predominant mechanism shortening the erythrocyte lifespan.
Depressed erythropoiesis is evidenced in our model by a transient suppression of reticulocyte counts despite adequate levels of circulating erythropoietin. The blunted response of the bone marrow to the erythropoietic hormone has been seen in other inflammatory mouse models, including a turpentine-induced sterile abscess model12 and a chronic interferon-γ (IFN-γ) overproduction model. Libregts et al33 demonstrated that IFN-γ stimulation promotes monocytic differentiation at the expense of erythroid differentiation, suggesting that erythroid suppression may be a side effect of the increased production of defensive myeloid cells during infection. Interestingly, we observed significantly increased leukocytosis in WT B abortus compared with Hamp-KO B abortus mice (supplemental Figure 3B). Following recent suggestions,33,35 we suspect that the increase in IFN-γ together with hepcidin-mediated iron restriction may promote erythropoietic suppression and a shift to leukocyte production. We speculate that delayed or inadequate shift to leukocyte production in Hamp-KO mice may have interfered with the neutralization of B abortus–associated pathogenic molecules, thereby increasing mortality. Although leukocytes are usually thought of as cells that mediate host defense against live microbes, a leukocyte defect that delayed the breakdown of heat-killed Aspergillus hyphae administered to mice worsened inflammatory tissue injury.36
Importantly, we were able to demonstrate that our mouse model of AI was partially hepcidin-dependent. The ablation of the hepcidin gene resulted in a dramatic reduction of iron-restricted erythropoiesis and improved mobilization of iron from tissue stores. At the nadir, the Hamp-KO mice had hemoglobin measurements that were 3 g/dL higher than in their WT counterparts, with a faster recovery to normal values. However, the Brucella-treated Hamp-KO mice were still markedly anemic at their nadir, with a hemoglobin level 5.8 g/dL below that of controls. Although hepcidin appears to play an important role in the development of AI in this model, other mechanisms contribute significantly.
The companion paper by Gardenghi et al34 shows strikingly similar findings with the same mouse model of AI. They observed a similar pattern of early hepcidin increases followed by marked iron-restricted anemia and a slow recovery. Using complementary studies, Gardenghi et al34 also demonstrated erythropoietic suppression and shortened erythrocyte lifespan. Flow cytometry confirmed an absence of early erythroid progenitor cells in the bone marrow, and a biotinylation assay showed an increased rate of erythrocyte elimination in the B abortus–treated mice. Hamp-KO mice in their experiments also had a milder anemia with an accelerated recovery. Erythrocyte survival in B abortus–treated Hamp-KO mice was shortened similarly to that of WT mice. Interestingly, Gardenghi et al34 found that the ablation of IL-6 also offered a protective effect against AI, albeit weaker than the effect of hepcidin-1 ablation.
As illustrated by its original use17 and the current studies by us and Gardenghi et al,34 the B abortus model of AI should be useful for the exploration of the mechanisms and possible treatments of AI.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Charles Lassman (Department of Pathology, UCLA) for his patient guidance in interpreting the histopathology sections. The authors also thank Drs Tara Arvedson, Barbra Sasu, and Keegan Cooke for introducing us to the mouse model described in this manuscript and for sharing the mouse hepcidin immunoassay. The authors thank all our colleagues at Xenon Pharmaceuticals for many helpful discussions that made this work possible.
This study was supported by research funding from Xenon Pharmaceuticals (to T.G. and E.N.).
Authorship
Contribution: A.K., E.F., E.V.V., E.N., and T.G. designed the studies, analyzed the data, and wrote or edited the manuscript; and A.K., E.F., S.G.P., E.V.V., and V.G. performed and analyzed the experiments.
Conflict-of-interest disclosure: T.G. and E.N. are cofounders, shareholders, and officers of Intrinsic LifeSciences, a company developing hepcidin diagnostics, and shareholders in Merganser Biotech, engaged in the development of hepcidin-based therapeutics. In the last 2 years, they have received research funding from Amgen and Xenon and were consultants for Xenon. T.G. is a consultant to Keryx. E.N. and T.G. hold patents related to the therapeutic use of hepcidin and its regulators. The remaining authors declare no competing financial interests.
Correspondence: Tomas Ganz, 37-055 CHS, Department of Medicine, UCLA, 10833 Le Conte Ave, Los Angeles, CA 90095; e-mail: tganz@mednet.ucla.edu.
References
Author notes
A.K. and E. F. contributed equally to this work.