Key Points
NOX-A12, a structured mirror-image RNA oligonucleotide that neutralizes CXCL12, interferes with CLL migration and drug resistance.
NOX-A12 inhibits chemotaxis and sensitizes CLL cells toward cytotoxic drugs, providing a rationale for NOX-A12 combination therapy.
The CXC chemokine ligand (CXCL12, or stromal cell-derived factor-1 as previously known) plays a critical role for homing and retention of chronic lymphocytic leukemia (CLL) cells in tissues such as the bone marrow (BM). In tissues, stromal cells constitutively secrete and present CXCL12 via cell-surface–bound glycosaminoglycans (GAGs), thereby attracting CLL cells and protecting them from cytotoxic drugs, a mechanism that may account for residual disease after conventional CLL therapy. NOX-A12, an RNA oligonucleotide in L-configuration (Spiegelmer) that binds and neutralizes CXCL12, was developed for interference with CXCL12 in the tumor microenvironment and for cell mobilization. Here, we examined effects of NOX-A12 on CLL cell migration and drug sensitivity. We found that NOX-A12 effectively inhibited CXCL12-induced chemotaxis of CLL cells. In contrast, NOX-A12 increased CLL migration underneath a confluent layer of BM stromal cells (BMSCs) due to interference with the CXCL12 gradient established by BMSCs. In particular, NOX-A12 competes with GAGs such as heparin for CXCL12 binding, leading to the release of CXCL12 from stromal cell-surface–bound GAGs, and thereby to neutralization of the chemokine. Furthermore, NOX-A12 sensitizes CLL cells toward bendamustine and fludarabine in BMSC cocultures. These data demonstrate that NOX-A12 effectively interferes with CLL cell migration and BMSC-mediated drug resistance, and establishes a rationale for clinical development of NOX-A12 in combination with conventional agents in CLL.
Introduction
Chronic lymphocytic leukemia (CLL), the most common adult leukemia in the Western hemisphere, is characterized by the expansion of CD5+CD23+ mature monoclonal B cells in the peripheral blood, lymph nodes, and the bone marrow (BM). To this day, CLL remains incurable with conventional chemoimmunotherapy, and although such therapies are highly effective in eliminating CLL cells in the peripheral blood, residual CLL cells often continue to persist in the BM and/or the lymph nodes. The tissue microenvironment provides survival and drug resistance signals to the CLL cells via soluble and cell-surface–bound factors such as the CXC chemokine ligand (CXCL12),1 BAFF and APRIL,2 and CD40 ligand (CD154).3 Therefore, disrupting the cross-talk between CLL cells and the stroma has become an area of drug development with the goal of interrupting CLL survival signaling pathways and sensitizing tissue CLL cells toward cytotoxic drugs, thereby reducing or eliminating residual disease.4
CLL cell migration and retention in the tissues is regulated by tissue gradients of chemokines which attract circulating CLL cells into the tissues through activation of corresponding chemokine receptors.5 In the BM, for example, BM stromal cells (BMSCs) constitutively secrete the chemokine CXCL12 and attract CLL cells via the CXCR4 chemokine receptor. CXCL12 production by BMSCs from CLL patients is increased under hypoxic (physiologic) oxygen concentration,6 an important finding that may explain how CXCL12 gradients are established within the BM microenvironment. The activity of CXCL12 is controlled and fine-tuned by glycosaminoglycans (GAGs), which sequester and present CXCL12 to CXCR4.7 CXCL12 binds with relative high affinity to GAGs on the surface of cells and the extracellular matrix, causing CXCL12-surface retention and exposure of the amino-terminal domain of CXCL12 for activation of CXCR4. Cell surface- or matrix-bound CXCL12 is thought to be the biologically most relevant form of CXCL12, based on in vitro7,8 and in vivo studies.9
Initial in vitro coculture studies in CLL demonstrated a chemoprotective effect of unselected BMSCs,10 and subsequent studies showed that diverse BMSCs of human and murine origin1,11 were highly effective in protecting CLL cells from both spontaneous- and drug-induced apoptosis. The protective effects of BMSCs require a close proximity between CLL and the stromal cells,10,-12 which is not surprising given that CLL cells display a high affinity for BMSCs, as exemplified by the striking in vitro phenomenon called pseudoemperipolesis (PEP).12 PEP describes the spontaneous migration of a fraction of CLL cells (or other leukemia cells) beneath BMSCs within a few hours (as shown by phase-contrast microscopy with the dark appearance of lymphocytes that migrated into the same focal plane as the stromal cells). Generally, PEP is used to describe symbiotic complexes of leukemia cells with their stromal cell component, caused by migration of leukemia cells beneath the adherent BMSCs.13 Besides attraction, as previously stated, BMSCs also protect CLL cells from spontaneous- and drug-induced apoptosis, which is largely dependent on close contact between CLL and the stromal cells. CXCL12 was originally known as pre–B-cell growth-stimulating factor,14 because it supported the proliferation of a stromal cell-dependent pre–B-cell clone, DW34. In CLL, CXCL12 also has direct prosurvival effects1,2 and it activates various signaling pathways, such as STAT3, AKT, and ERK1/2.1,15,16
Due to the importance of the CXCL12-CXCR4 axis in CLL, previous preclinical15 and clinical studies17 focused on the blockade of CXCR4 with small molecule CXCR4 antagonists, which were the first drugs available for inhibition of the CXCL12-CXCR4 axis.4 A phase 1 clinical trial of plerixafor in combination with the anti-CD20 antibody rituximab in relapsed CLL suggested a plerixafor dose-dependent mobilization of CLL cells into the blood.17 Other plerixafor trials for chemosensitization in patients with leukemia and lymphoma are ongoing, along with trials in cancer patients using alternative CXCR4-targeting agents, such as peptide CXCR4 antagonists or anti-CXCR4 mAbs.18 Targeting the other part of this axis, the chemokine CXCL12, represents an attractive alternative approach; however, given the extremely high evolutionary conservation of CXCL12, raising efficacious antibodies against CXCL12 has been difficult. But Spiegelmer technology can bypass such obstacles by providing mirror-image oligonucleotides that specifically bind to proteins in a manner similar to antibodies.19 Spiegelmers are RNA oligonucleotides in an L-configuration (ie, the mirror image of naturally occurring RNA), and designed to bind target molecules with high affinity and specificity.20,21 Due to their mirror-image configuration, Spiegelmers are not susceptible to degradation by nucleases and do not hybridize with native nucleic acids. Furthermore, they are immunologically passive, in that, there is no antibody formation or toll-like receptor activation. NOX-A12 is a Spiegelmer that binds and antagonizes CXCL12. After completion of a clinical phase 1 trial in healthy volunteers (study #SNOXA12C001),22 NOX-A12 is currently being tested in an ongoing phase 2a clinical trial in combination with chemoimmunotherapy (bendamustine and rituximab) in patients with relapsed CLL (ClinicalTrials.gov, #NCT01486797), and in combination with bortezomib and dexamethasone in patients with relapsed multiple myeloma (#NCT01521533). Preclinical activity of NOX-A12 in functional assays has not previously been tested with regard to CLL; therefore, we investigated the effects of NOX-A12 on CXCL12-related CLL cell functions and drug sensitivity in model systems that provide CXCL12 stimulation.
Methods
NOX-A12, revNOX-A12, cell purification, and cell lines
NOX-A12 was chemically synthesized as described,23 lyophilized, and dissolved in sterile ultrapure water as stock concentration of 1 mM for storage at −20°C until use. For CLL cell isolation, peripheral blood samples were obtained with informed consent from patients fulfilling diagnostic and immunophenotypic criteria for CLL at the Department of Leukemia at MD Anderson Cancer Center. Patient consent was obtained in accordance with the Declaration of Helsinki on protocols that were reviewed and approved by the MD Anderson Cancer Center’s Institutional Review Board. Peripheral blood mononuclear cells were isolated via density gradient centrifugation over Ficoll-Paque (GE Healthcare Life Sciences), and used fresh or viably frozen in fetal bovine serum (FBS; SAFC Biosciences), plus 10% dimethylsulfoxide (Sigma-Aldrich) for storage in liquid nitrogen. All assays with primary CLL cells contained more than 88% CD19+ B cells and were exclusively performed at MD Anderson Cancer Center. The lymphoid cell lines obtained from the DSMZ (Brauschweig, Germany) were used at NOXXON. The murine stromal cell line 9-15C derived from BM was purchased from the Riken Cell Bank and maintained in RPMI 1640 (medium) supplemented with 2.05 mM L-Gln (HyClone), 10% FBS (SAFC Biosciences), and penicillin-streptomycin (CellGro). The murine stromal cell line TSt-4, derived from fetal thymus tissue (also from the Riken Cell Bank) was maintained in RPMI 1640 supplemented with 2.05 mM L-Gln, 5% FBS, and penicillin-streptomycin. Murine cell lines could be used due to cross-reactivity of murine CXCL12 with both NOX-A12 and human CXCR4 on lymphoid cells.
CLL chemotaxis assays
Chemotaxis assay across polycarbonate Transwell culture inserts (Costar; Corning, NY) was performed as previously described.12 Briefly, CLL samples were suspended to a concentration of 107 cells per mL in RPMI 1640 with 0.5% bovine serum albumin in the presence or absence of the CXCR4 antagonist AMD3100 (Sigma-Aldrich), and a total of 100 μL containing 106 cells was added to the top chamber of a Transwell culture insert with a diameter of 6.5 mm and a pore size of 5 μm. Filters were then transferred to wells containing medium, with or without 200 ng/mL CXCL12 (Upstate Biotechnology), and different concentrations of NOX-A12 (3 nM to 300 nM). The chambers were incubated for 3 hours at 37°C in 5% CO2 and the cells in the lower chamber were suspended and divided into aliquots for counting with a FACSCalibur for 20 seconds at 60 μL/min in duplicates. A 1/20 dilution of input cells was counted under the same conditions.
Chemotaxis with lymphoid cell lines
Jurkat cells and Nalm-6 cells were cultivated overnight at a cell density of 0.3 × 106/mL and 0.75 × 106/mL respectively, at 37°C, and 5% CO2 in RPMI 1640 was supplemented with GlutaMAX (Invitrogen), 10% FBS, 50 U/mL penicillin, and 50 µg/mL streptomycin. Also, 0.3 nM of recombinant CXCL12 was preincubated with various concentrations of NOX-A12 in the lower compartments of a 96-well Corning Transwell plate with 5 µm pores (#3388; Costar) at 37°C for 20 to 30 minutes. The stimulation solutions were prepared in Hank’s Balanced Salt Solution including 0.1% bovine serum albumin and 20 mM HEPES (HBH). The cells were washed in HBH and 1 × 105 cells were added to the insert of the Transwell. The cells were then allowed to migrate for 3 hours at 37°C. After incubation, the insert plates were removed and 30 μL resazurin working solution (440 μM in phosphate-buffered saline [PBS]) was added to the lower wells. The plates were then incubated at 37°C for 2.5 hours and 100 μL of each well were transferred to a black 96-well plate for measurement of the relative fluorescence in a plate reader (Synergy 2; Biotek). The relative fluorescence was normalized (chemotaxis to 0.3 nM; CXCL12 was set to 100%), plotted against NOX-A12 concentration, and the IC50-value was determined using GraphPad Prism software.
Migration assays beneath BMSC
To evaluate the impact of NOX-A12 on migration of CLL cells beneath BMSCs (PEP), we quantified CLL cell PEP as described.12 Briefly, the murine stromal cell lines TSt-4 and 9-15C were seeded the day before the assay onto collagen-coated, 12-well plates at a concentration per well of 1.8 × 105 cells per mL, in RPMI 1640 supplemented with 10% fetal calf serum and penicillin-streptomycin-Gln. The next day, BMSC layers were washed with RPMI 1640 and incubated for 1 hour with 100 nM NOX-A12 or 200 ng/mL human CXCL12. The CLL cells were suspended at a concentration of 107 cells per mL in medium and added to the stromal cell layers in the presence or absence of AMD3100, and incubated at 37°C in 5% CO2 for 4 hours. Cells that had not migrated into the stromal cell layer were removed by vigorously washing with RPMI 1640. The complete removal of nonmigrated cells and the integrity of the stromal cell layer containing transmigrated cells were assessed by phase-contrast microscopy and documented photographically. The stromal cell layer containing transmigrated cells was detached by incubation for 1 minute with trypsin/EDTA and prewarmed to 37°C (Gibco-BRL). Cells were then immediately suspended by adding 1 mL of ice-cold RPMI 1640 to 10% FBS, washed, and suspended in 0.4 mL of cold medium for counting by flow cytometry for 20 seconds at 60 µL/min, in duplicates. A lymphocyte gate was set according to the different relative size and granularity (forward scatter and side scatter) characteristics to exclude stromal cells from the counts. The number of migrated cells under each condition was expressed as percentage of the control. In another set of experiments, 1.5 × 104 murine stromal MS-5 cells (DSMZ) were seeded in 100 μL MEM-α supplemented with 10% FBS, 50 U/mL penicillin, and 50 µg/mL streptomycin in black 96-well plates with clear bottoms and incubated overnight at 37°C with 5% CO2. The next day, the cells were washed with 200 µL RPMI 1640 and incubated for 24 hours in 100 µL RPMI 1640 (plus 1% FBS) at 37°C. Various concentrations of NOX-A12 and AMD3100 were added to the wells and incubated for 30 minutes. Jurkat and Nalm-6 cells were incubated with 5 µM Calcein-AM (Invitrogen) for 30 minutes and washed twice with RPMI 1640; and 1.5 × 105 Jurkat or 3.5 × 105 Nalm-6 cells, respectively, were added to the stromal cell layer and incubated for 3 or 6 hours, respectively. Bottom fluorescence (Ex/Em 485 nm/528 nm) was measured using a plate reader (Synergy 2) before and after six washes with 100 µL RPMI 1640. The quotients of the fluorescence values before and after washing were calculated, and the percentages of migrated and attached cells were plotted against the concentration of NOX-A12.
CXCL12 detachment assay
Murine stromal cells (MS-5, 9-15C, and TSt-4) were seeded in 24- or 96-well plates at a density of 1 × 105 or 1.5 × 104 per well, respectively, in MEM-α (MS-5) or RPMI 1640 (9-15C, TSt-4) with 10% FBS. The next day, the cells were washed and 0.5 mL or 0.1 mL of fresh RPMI 1640 with 1% FBS was added. Cells were incubated for 72 hours and NOX-A12 or heparin (from porcine intestinal mucosa, #H3149; Sigma-Aldrich) was added for the indicated time points. CXCL12 released by the stromal cells was quantified by enzyme-linked immunosorbent assay (ELISA) for murine CXCL12 (#MCX120; R&D Systems) or human CXCL12 that also detects CXCL12 produced by murine stromal cells (#DSA00; R&D Systems) according to the manufacturer’s recommendations.
Quantitative reverse-transcription polymerase chain reaction for CXCL12
MS-5 cells were treated with 100 nM NOX-A12 for the indicated time periods prior to RNA extraction using the RNeasy Mini Kit (Qiagen), followed by subsequent cDNA preparation, polymerase chain reaction (PCR) amplification using the OneStep RT-PCR Kit (Qiagen), and the following primers: ATGAACGCCAAGGTCGTGGTC and TGGCTGTTGTGCTTACTTGTTT. PCR-amplified DNA was quantified for the indicated PCR cycles using the Quant-iT PicoGreen dsDNA Reagent (Invitrogen) according to the manufacturer’s recommendations.
Intracellular CXCL12 staining
MS-5 cells were seeded in 6-well plates at a density per well of 1 × 106 in 3 mL MEM-α which also contained 10% FBS. The next day, the cells were washed and incubated for 1 hour with 3 mL fresh medium with 1% FBS containing 100 nM NOXA12 or 100 nM of the nonfunctional control revNOX-A12 (reversed sequence). Cells were washed twice with PBS and incubated with PBS-based Cell Dissociation Buffer (#13151-014; Gibco). Cells were fixed with Fixation Buffer (#FC004; R&D Systems) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (#FC005; R&D Systems). Intracellular CXCL12-specific staining was performed with a monoclonal CXCL12-specific APC-labeled antibody (clone #79018; R&D Systems) and compared with the correspondent APC-labeled isotype control (clone#11711; R&D Systems). Flow cytometry analyses were performed using the Guava easyCyte 6HT-2L instrument and histograms were generated using InCyte software (Millipore).
Biacore measurements
Biotinylated heparin (#B9806; Sigma-Aldrich) was immobilized on a neutravidin-coated carboxydextran (CM5) chip. The reference flow cell was blocked with biotin. All reagents for Biacore measurements were purchased from GE Healthcare unless otherwise specified. Binding of human CXCL12/SDF-1a (R&D Systems) to an immobilized heparin surface was analyzed by injecting a concentration series of CXCL12 for 240 seconds in running buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, and 1 mM MgCl2). At least one concentration was injected twice to monitor regeneration efficiency and flow cell integrity. All experiments were performed at 37°C and a flow of 30 µL/min. The assay was double referenced, whereas the reference flow cell served as surface control and a series of buffer injections without analyte determined the bulk contribution of the buffer itself on both flow cells. To address the binding of CXCL12 in the context of NOX-A12, a competitive Biacore assay was set up. Immobilization of biotinylated heparin was performed as described above. CXCL12 was injected at a fixed concentration of 125 nM, together with a concentration series of NOX-A12 or soluble heparin from porcine intestinal mucosa (#H3149; Sigma-Aldrich) itself. In both experimental designs, the response units after 240 seconds of injection were determined and plotted using GraphPad Prism Software. Detachment of heparin-bound CXCL12 by NOX-A12 was measured by injections of 100 nM of NOX-12, 100 nM reverse NOX-A12 (revNOX-A2), or running buffer during the dissociation phase of 2.5 µM CXCL12 from immobilized heparin. Response units at predefined report points recorded data directly after the association phase of CXCL12 binding to heparin and after 240 seconds of the dissociation phase in the presence of buffer, NOX-A12, or revNOX-A12. Each experiment was repeated at least 3 times.
Chemosensitization assay
For chemosensitization experiments, 9-15C stromal cells were detached with enzyme-free cell dissociation buffer (Life Technologies) and incubated in the presence or absence of 200 nM NOX-A12 in RPMI 1640 with 1% FBS for 1 hour while rotating at 37°C in 5% CO2. After incubation, cells were washed once with medium and then seeded into 24-well plates (Corning Life Sciences) at a concentration per well of 5 × 106 cells per mL in the presence or absence of 200 nM NOX-A12. CLL cells were added immediately onto the stromal cells, and after 30 minutes, 10 μM bendamustine (Sigma-Aldrich) and 10 μM arabinosyl-2-fluoroadenine (F-ara-A; Sigma-Aldrich) were added. After 24 hours, the CLL cells were collected by washing them off, and then assayed for cell viability. Determination of CLL cell viability was based on the analysis of mitochondrial transmembrane potential by 3,3′-dihexyloxacarbocyanine iodide (DiOC6) (Molecular Probes; Invitrogen) and cell membrane permeability to propidium iodide, as described.11
Results
NOX-A12 inhibits chemotaxis of CLL cells and lymphoid cell lines toward CXCL12
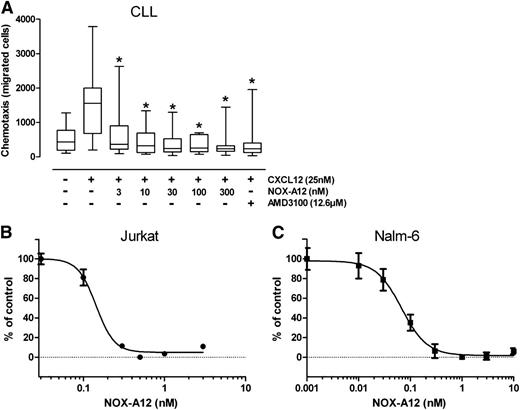
As shown in Figure 1A, CLL cell chemotaxis toward 200 ng/mL (25 nM) of CXCL12 was significantly inhibited by NOX-A12 in a dose-dependent manner in samples from 12 different CLL patients. The results demonstrated a significant reduction in CLL migration toward CXCL12, already at a very low NOX-A12 concentration of 3 nM, where the median of CLL cells migrating toward CXCL12 decreased significantly from 1556 to 367. The CXCR4 receptor antagonist AMD3100 served as internal control, of which a much higher concentration (12.6 µM) than NOX-A12 was necessary to inhibit CXCL12-mediated and CXCR4-dependent chemotaxis of CLL cells. In the T-cell line Jurkat and the acute lymphoid leukemia (ALL) cell line Nalm-6 (Figure 1B-C), both of which migrate toward CXCL12 with high sensitivity, NOX-A12–mediated inhibition of chemotaxis was observed with a subnanomolar IC50.
NOX-A12 inhibits CXCL12-dependent chemotaxis of primary CLL cells and cell lines. (A) NOX-A12 significantly inhibited chemotaxis of primary CLL cells toward CXCL12. CLL cells from 12 different patients were allowed to migrate toward 25 nM CXCL12 that was preincubated with different concentrations of NOX-A12. The box plots represent the median including interquartile range, maximum, and minimum of migrated CLL cells. The Wilcoxon matched-pairs signed-ranks test was used for statistical description. (B) The acute T-cell leukemic cell line Jurkat, and (C) the pre-B ALL cell line Nalm-6 were assayed for chemotaxis toward CXCL12. Results indicate relative migration compared with control samples migrating to 0.3 nM CXCL12 and samples preincubated with different concentrations of NOX-A12, representing the mean ± SD values (n = 3). Data are representative of 3 or more independent experiments.
NOX-A12 inhibits CXCL12-dependent chemotaxis of primary CLL cells and cell lines. (A) NOX-A12 significantly inhibited chemotaxis of primary CLL cells toward CXCL12. CLL cells from 12 different patients were allowed to migrate toward 25 nM CXCL12 that was preincubated with different concentrations of NOX-A12. The box plots represent the median including interquartile range, maximum, and minimum of migrated CLL cells. The Wilcoxon matched-pairs signed-ranks test was used for statistical description. (B) The acute T-cell leukemic cell line Jurkat, and (C) the pre-B ALL cell line Nalm-6 were assayed for chemotaxis toward CXCL12. Results indicate relative migration compared with control samples migrating to 0.3 nM CXCL12 and samples preincubated with different concentrations of NOX-A12, representing the mean ± SD values (n = 3). Data are representative of 3 or more independent experiments.
NOX-A12 increases PEP of CLL cells and lymphoid cell lines
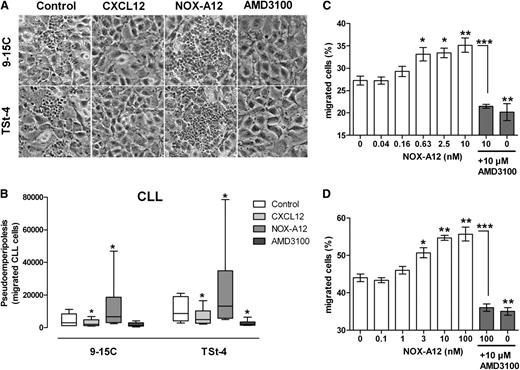
We next analyzed the effects of NOX-A12 on spontaneous migration of CLL cells beneath BMSCs (PEP).12,15 Surprisingly, and in contrast to NOX-A12–inhibited chemotaxis, we noted an increased migration of CLL cells beneath BMSCs in the presence of NOX-A12. Phase-contrast photomicrographs (Figure 2A) show a representative CLL sample cocultured with 9-15C and TSt-4 stromal cells in the presence of CXCL12, NOX-A12, or AMD3100. The data demonstrates a marked increase in CLL cell migration beneath BMSCs after pretreatment with NOX-A12, and robust inhibition of PEP by CXCL12 and AMD3100, as previously described.15 In samples from 6 patients, pretreatment with 100 nM NOX-A12 significantly increased the number of migrated CLL cells from a median of 2994 to 6641 using 9-15C cells, and from 8599 to 13 161 using TSt-4 cells (Figure 2B). Treatment with the CXCR4 antagonist AMD3100 and CXCL12 significantly inhibited the migration beneath BMSCs. In accordance with these findings, NOX-A12 also promoted PEP of Jurkat and Nalm-6 cells beneath MS-5 BMSC layers (Figure 2C-D) in a dose-dependent fashion, which was inhibited by AMD3100. These opposite effects of NOX-A12 and AMD3100 on CLL cell migration in the PEP assays, but not in the chemotaxis assays, reflect different mechanisms of action of NOX-A12 and AMD3100, acting via CXCL12 neutralization or CXCR4 antagonism, respectively; we have explored reasons for this difference in the following assays.
NOX-A12 increases CLL cell migration beneath BMSCs and induces migration of leukemic cell lines in a dose-dependent manner. (A) A representative phase-contrast photomicrograph of CLL cells migrated underneath stromal cells 9-15C and TSt-4 (control), and in comparison, increased migration of the same CLL sample after incubation of the stromal cells with 100 nM NOX-A12. (B) CLL cells that migrated underneath stromal cells were quantified by flow cytometry. The box-and-whisker diagrams represent the median including interquartile range, maximum, and minimum of migrated CLL cells from 6 different patients after pretreatment of the stromal cells with or without NOX-A12. The Wilcoxon matched-pairs signed-rank test was used for statistical analysis. (C) The T-cell leukemic cell line Jurkat, and (D) the ALL cell line Nalm-6 were labeled with Calcein AM and cocultured with murine stromal MS-5 cells preincubated with different concentrations of NOX-A12 and/or AMD3100. After incubation of 3 (Jurkat) or 6 (Nalm-6) hours, respectively, leukemia cells were washed from the stromal cell layer. Results indicate the percentages of migrated plus attached cells beneath the stromal cell layer, representing the mean ± SD values of bottom fluorescence measurement (n = 3). The one-way analysis of variance test was conducted for statistical analysis. Data are representative of 3 independent experiments. *P = .01 to .05; **P = .001 to .01; ***P < .001.
NOX-A12 increases CLL cell migration beneath BMSCs and induces migration of leukemic cell lines in a dose-dependent manner. (A) A representative phase-contrast photomicrograph of CLL cells migrated underneath stromal cells 9-15C and TSt-4 (control), and in comparison, increased migration of the same CLL sample after incubation of the stromal cells with 100 nM NOX-A12. (B) CLL cells that migrated underneath stromal cells were quantified by flow cytometry. The box-and-whisker diagrams represent the median including interquartile range, maximum, and minimum of migrated CLL cells from 6 different patients after pretreatment of the stromal cells with or without NOX-A12. The Wilcoxon matched-pairs signed-rank test was used for statistical analysis. (C) The T-cell leukemic cell line Jurkat, and (D) the ALL cell line Nalm-6 were labeled with Calcein AM and cocultured with murine stromal MS-5 cells preincubated with different concentrations of NOX-A12 and/or AMD3100. After incubation of 3 (Jurkat) or 6 (Nalm-6) hours, respectively, leukemia cells were washed from the stromal cell layer. Results indicate the percentages of migrated plus attached cells beneath the stromal cell layer, representing the mean ± SD values of bottom fluorescence measurement (n = 3). The one-way analysis of variance test was conducted for statistical analysis. Data are representative of 3 independent experiments. *P = .01 to .05; **P = .001 to .01; ***P < .001.
NOX-A12 induces release of CXCL12 from murine stromal cell lines
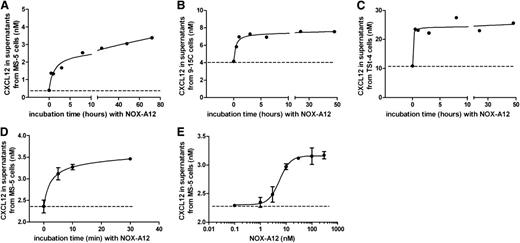
We hypothesized that the NOX-A12–promoted increase in PEP of CLL cells might be the result of NOX-A12–induced CXCL12 release from the stromal cells. Therefore, we tested the effect of NOX-A12 on the CXCL12 release using 3 different BMSC lines (MS-5, 9-15C, and TSt-4). We found that NOX-A12 induced rapid CXCL12 release into the supernatant of all 3 cell lines (Figure 3A-C). The basal secretion levels of CXCL12 were highest in TSt-4 cells, followed by 9-15C and MS-5 cells. The observed increase of CXCL12 levels was very rapid and occurred within 5 minutes of incubation with NOX-A12 (Figure 3D). NOX-A12–dependent release of CXCL12 in MS-5 cells was achieved with an EC50 of approximately 5 nM (Figure 3E). CXCL12 transcript levels were not altered by NOX-A12 incubation over a period of 3 days, as shown by quantitative reverse-transcription polymerase chain reaction. In addition, staining for cytoplasmatic CXCL12 using flow cytometry did not reveal any significant changes when BMSCs were incubated with NOX-A12 (supplemental Figure 1, available on the Blood Web site). Therefore, it seems that CXCL12 is released from extracellular storages of BMSCs.
NOX-A12 induces release of CXCL12 from murine stromal cell lines. (A-C) The murine BMSCs MS-5, 9-15C, and TSt-4 were incubated with 100 nM NOX-A12 for the indicated time spans leading to the release of CXCL12. The dashed lines indicate the basal CXCL12 secretion levels. (D) CXCL12 was rapidly released within 5 minutes after incubation with 100 nM NOX-A12. (E) MS-5 cells were incubated with various concentrations of NOX-A12 for 30 minutes. Results indicate the concentration of CXCL12 in supernatants quantified by ELISA. Data are representative of 3 independent experiments.
NOX-A12 induces release of CXCL12 from murine stromal cell lines. (A-C) The murine BMSCs MS-5, 9-15C, and TSt-4 were incubated with 100 nM NOX-A12 for the indicated time spans leading to the release of CXCL12. The dashed lines indicate the basal CXCL12 secretion levels. (D) CXCL12 was rapidly released within 5 minutes after incubation with 100 nM NOX-A12. (E) MS-5 cells were incubated with various concentrations of NOX-A12 for 30 minutes. Results indicate the concentration of CXCL12 in supernatants quantified by ELISA. Data are representative of 3 independent experiments.
Recombinant CXCL12 binds to the surface of MS-5 stromal cells, previously stripped of endogenous CXCL12 by NOX-A12
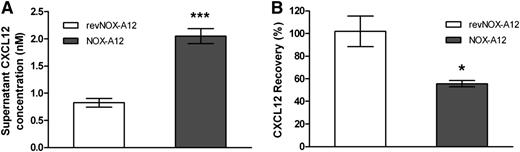
Next, we determined that CXCL12 is bound to the extracellular cell surface and is being detached by NOX-A12. We first incubated MS-5 stromal cells with NOX-A12, which led to an increase of CXCL12 in the supernatant (Figure 4A), followed by a wash step in order to remove the CXCL12/NOX-A12 complexes. The subsequent addition of recombinant CXCL12 led to a decrease of CXCL12 levels in the supernatant (Figure 4B), indicating that CXCL12 bound to stromal cells that were preincubated with NOX-A12 but not with a nonfunctional control (revNOX-A12). The fact that no decrease in CXCL12 was observed when the chemokine was added to nontreated cells indicated that the cell surface of MS-5 stromal cells was saturated with endogenous CXCL12. In summary, this finding indicates that NOX-A12 is able to strip off CXCL12 from the cell surface.
Recombinant CXCL12 binds to the surface of MS-5 stromal cells previously stripped of endogenous CXCL12 by NOX-A12. (A) MS-5 cells were incubated with 100 nM NOX-A12 or with the inactive variant revNOX-A12 for 1 hour. CXCL12 concentrations in supernatants were quantified by ELISA. Extracellular CXCL12 most likely presented by GAGs was detached from MS-5 cells by NOX-A12. (B) MS-5 cells were washed three times to remove any free NOX-A12 and incubated with 1 nM recombinant human CXCL12 for 3 hours. CXCL12 concentrations in supernatants were quantified by ELISA and the percentage of CXCL12 recovered from supernatants was calculated. The results indicate that recombinant CXCL12 binds to the extracellular binding sites of MS-5 cells that were previously stripped of endogenous CXCL12 by NOX-A12. The unpaired Student t test was used for statistical analysis. Data are representative of 3 independent experiments. *P = .01 to .05; **P = .001 to .01; ***P < .001.
Recombinant CXCL12 binds to the surface of MS-5 stromal cells previously stripped of endogenous CXCL12 by NOX-A12. (A) MS-5 cells were incubated with 100 nM NOX-A12 or with the inactive variant revNOX-A12 for 1 hour. CXCL12 concentrations in supernatants were quantified by ELISA. Extracellular CXCL12 most likely presented by GAGs was detached from MS-5 cells by NOX-A12. (B) MS-5 cells were washed three times to remove any free NOX-A12 and incubated with 1 nM recombinant human CXCL12 for 3 hours. CXCL12 concentrations in supernatants were quantified by ELISA and the percentage of CXCL12 recovered from supernatants was calculated. The results indicate that recombinant CXCL12 binds to the extracellular binding sites of MS-5 cells that were previously stripped of endogenous CXCL12 by NOX-A12. The unpaired Student t test was used for statistical analysis. Data are representative of 3 independent experiments. *P = .01 to .05; **P = .001 to .01; ***P < .001.
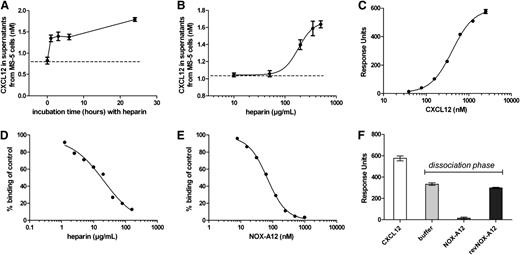
NOX-A12 competes with the GAG heparin for binding to CXCL12
CXCL12 is bound to GAG such as heparin on the surface of cells and this causes CXCL12 surface retention.7 To confirm that CXCL12 is bound to the extracellular surface and is being detached by NOX-A12, we incubated the MS-5 stromal cells with the GAG heparin, which also led to the release of CXCL12 in a time- and dose-dependent manner (Figure 5A-B). Of note, compared with NOX-A12, much higher concentrations were needed. To corroborate the mode-of-action of these findings, we determined the interaction of human CXCL12 with an immobilized heparin surface by Biacore measurement. CXCL12 bound to immobilized heparin with an affinity of 407 nM with fast-determined association and dissociation rate constants (Figure 5C). Both NOX-A12 and heparin itself were able to compete the binding of CXCL12 to immobilized heparin in solution (Figure 5D-E). In a Biacore flow system, NOX-A12 detached heparin-bound CXCL12, while the nonfunctional control revNOX-A12 showed no influence on the detachment of CXCL12, comparable to buffer (Figure 5F). These observations revealed that the NOX-A12 binding site to CXCL12 is most likely in close proximity or overlaps the heparin-binding site of CXCL12. Thus, competition of CXCL12 binding to heparin with NOX-A12 reflects the mode-of-action that is responsible for the CXCL12 detachment from extracellular GAGs.
NOX-A12 competes with the GAG heparin for binding to CXCL12. (A) MS-5 cells were incubated with high concentrations of heparin (200 µg/mL) for the indicated time leading to the release of CXCL12. Results indicate the concentration of CXCL12 in supernatants quantified by ELISA. The dashed line indicates the basal CXCL12 secretion levels. (B) MS-5 cells were incubated with various concentrations of heparin for 3 hours. Results indicate the concentration of CXCL12 in supernatants quantified by ELISA. Heparin induced a concentration-dependent release of CXCL12 in MS-5 cells with an EC50 of about 180 µg/mL (12.4 µM based on a molecular weight of 14.5 kilodalton). (C) CXCL12 showed a dose-dependent binding to immobilized biotinylated heparin with dissociation constant (KD) of 407 nM. (D) Simultaneous injection of 125 nM of CXCL12 in the presence of various heparin concentrations in solution led to a dose-dependent competition of CXCL12 binding to the immobilized heparin with an IC50 of 21 µg/mL (1.45 µM based on a molecular weight of 14.5 kilodalton). (E) Simultaneous injection of 125 nM of CXCL12 together with NOX-A12 led to a competition of CXCL12 binding to immobilized heparin with an IC50 of 67.93 nM. (F) NOX-A12 mobilized heparin-bound CXCL12 in a Biacore flow system. Injection of 2.5 µM of human CXCL12 led to binding to immobilized heparin. Injection of 100 nM of NOX-A12 for 30 seconds during the dissociation phase of the binding event led to a release of heparin-bound CXCL12 from the surface, whereas revNOX-A12 showed no significant influence on the dissociation of CXCL12 from immobilized heparin compared with buffer.
NOX-A12 competes with the GAG heparin for binding to CXCL12. (A) MS-5 cells were incubated with high concentrations of heparin (200 µg/mL) for the indicated time leading to the release of CXCL12. Results indicate the concentration of CXCL12 in supernatants quantified by ELISA. The dashed line indicates the basal CXCL12 secretion levels. (B) MS-5 cells were incubated with various concentrations of heparin for 3 hours. Results indicate the concentration of CXCL12 in supernatants quantified by ELISA. Heparin induced a concentration-dependent release of CXCL12 in MS-5 cells with an EC50 of about 180 µg/mL (12.4 µM based on a molecular weight of 14.5 kilodalton). (C) CXCL12 showed a dose-dependent binding to immobilized biotinylated heparin with dissociation constant (KD) of 407 nM. (D) Simultaneous injection of 125 nM of CXCL12 in the presence of various heparin concentrations in solution led to a dose-dependent competition of CXCL12 binding to the immobilized heparin with an IC50 of 21 µg/mL (1.45 µM based on a molecular weight of 14.5 kilodalton). (E) Simultaneous injection of 125 nM of CXCL12 together with NOX-A12 led to a competition of CXCL12 binding to immobilized heparin with an IC50 of 67.93 nM. (F) NOX-A12 mobilized heparin-bound CXCL12 in a Biacore flow system. Injection of 2.5 µM of human CXCL12 led to binding to immobilized heparin. Injection of 100 nM of NOX-A12 for 30 seconds during the dissociation phase of the binding event led to a release of heparin-bound CXCL12 from the surface, whereas revNOX-A12 showed no significant influence on the dissociation of CXCL12 from immobilized heparin compared with buffer.
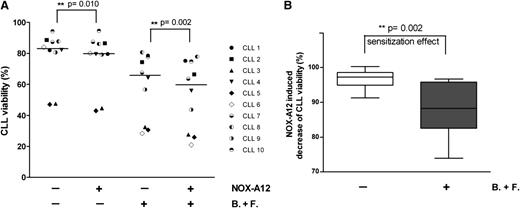
NOX-A12 chemosensitizes CLL cells toward cytotoxic agents in coculture with BMSCs
BMSCs protect CLL cells from drug-induced apoptosis.11,15 To test the ability of NOX-A12 to chemosensitize CLL cells, the latter were plated onto NOX-A12 pretreated BMSCs, which were therefore CXCL12-stripped, or control BMSCs, which still presented CXCL12 on the cell surface. Subsequently, the coculture was exposed to cytotoxic drugs (a combination of bendamustine and fludarabine/F-AraA). As shown in Figure 6A, there was a modest, yet significant decrease in CLL cell viability after NOX-A12 treatment. The expected reduction in CLL cell viability after exposure to the cytotoxic agents bendamustine and fludarabine/F-AraA was significantly enhanced by NOX-A12, suggesting a significant level of chemosensitization (Figure 6B), which provides a rational basis for the ongoing CLL trial with NOX-A12.
NOX-A12 chemosensitizes CLL cells toward cytotoxic agents in cocultures with BMSCs. (A) CXCL12 was stripped of BMSCs before CLL cells were cocultured with BMSCs in medium alone (control) or in medium containing NOX-A12, the combination of bendamustine and fludarabine (B+F), or NOX-A12 combined with B+F. CLL cell viability was determined after 24 hours by staining with DiOC6 and propidium iodide flow cytometry. Dark lines represent the median of CLL viability for 10 different patients. Statistic differences were determined by the Wilcoxon matched-pairs signed-rank test. (B) The box diagram represents the mean relative viabilities of CLL cells cocultured with BMSCs and treated with NOX-A12 with or without bendamustine and fludarabine. Viabilities of NOX-A12–treated samples were normalized to the viabilities of control samples without NOX-A12 (100%). The Wilcoxon matched-pairs signed-rank test was used to demonstrate the NOX-A12–induced sensitization effect for the chemotherapeutic drugs.
NOX-A12 chemosensitizes CLL cells toward cytotoxic agents in cocultures with BMSCs. (A) CXCL12 was stripped of BMSCs before CLL cells were cocultured with BMSCs in medium alone (control) or in medium containing NOX-A12, the combination of bendamustine and fludarabine (B+F), or NOX-A12 combined with B+F. CLL cell viability was determined after 24 hours by staining with DiOC6 and propidium iodide flow cytometry. Dark lines represent the median of CLL viability for 10 different patients. Statistic differences were determined by the Wilcoxon matched-pairs signed-rank test. (B) The box diagram represents the mean relative viabilities of CLL cells cocultured with BMSCs and treated with NOX-A12 with or without bendamustine and fludarabine. Viabilities of NOX-A12–treated samples were normalized to the viabilities of control samples without NOX-A12 (100%). The Wilcoxon matched-pairs signed-rank test was used to demonstrate the NOX-A12–induced sensitization effect for the chemotherapeutic drugs.
Discussion
This study provides evidence for a unique, novel mechanism-of-action of the CXCL12-neutralizing Spiegelmer NOX-A12 in CLL models, with broader implications for NOX-A12–mediated effects on cell migration and chemosensitization. NOX-A12 inhibits CXCL12-directed chemotaxis of CLL cells with high potency, but surprisingly, we also noticed that NOX-A12 promoted PEP of CLL cells in vitro. These opposite effects of NOX-A12 on cell migration were confirmed using lymphoid cell lines, Jurkat and Nalm-6. We interpreted these findings as a reflection of different complexity in the in vitro assays. In the Boyden chamber chemotaxis assays, synthetic CXCL12 in the lower chambers of the assay was neutralized by NOX-A12; thereby, the CXCL12 gradient was abrogated. Consequently, CLL cell migration in this assay was largely inhibited (Figure 1). In contrast, in the PEP assay, stromal cell-derived CXCL12 was immobilized on the surface of the BMSCs. Surface-bound CXCL12 was stripped off by NOX-A12 and released into the supernatant (Figure 3), and consequently neutralized. Specifically, the NOX-A12–binding site to CXCL12, which likely is in close proximity to or overlapping with the heparin-binding site of CXCL12, competes with CXCL12 binding to heparin (Figure 5) leading to the release of GAG-bound CXCL12 (Figures 3 and 4). Due to this removal of surface-bound CXCL12, the gradient toward high CXCL12 concentrations underneath the BMSC layer increases, which in turn attracts larger numbers of CLL cells (Figure 2). When compared with murine BMSCs, primary human BMSC and human BMSC lines secreted much lower or undetectable levels of CXCL12 that were not sufficient for inducing PEP of CLL cells (data not shown). Therefore, such cells do not appear to be suitable for testing a CXCL12 antagonist such as NOX-A12. The reason for this comparably low CXCL12 secretion by human BMSCs in vitro is unknown, but it could be related to greater heterogeneity and age of human BMSCs when compared with murine BMSCs.
Because these attracted CLL cells are protected from chemotherapeutic agents due to positive CXCL12 survival signals for example, we did not observe a sensitization effect of NOX-A12 in this standard 2D coculture assay (data not shown). Therefore, we developed a novel coculture approach adapted for CXCL12 antagonists. We first stripped off CXCL12 from the stromal cell surface, and subsequently cocultured CLL cells with either the CXCL12-depleted or CXCL12-containing stromal cells. We observed a slight significant NOX-A12–mediated decrease of CLL viability (Figure 6A), which is in line with previously reported positive CXCL12 effects on CLL cells.1 More importantly, when combined with chemotherapeutic drugs, the NOX-A12 effect on CLL cell viability significantly increased suggesting a chemosensitization effect (Figure 6B). In vivo, NOX-A12 abrogates the peripheral BM-directed CXCL12 gradient, which leads to mobilization of hematopoietic stem cells, as well as other cell types.22 Because lymphocytes are effectively mobilized in healthy volunteers,22 CLL cells, which are aberrant lymphocytes with high CXCR4 expression, should also be effectively mobilized with NOX-A12. This is supported by preliminary data from an ongoing clinical trial in relapsed CLL patients.24
Collectively, this study suggests that competition of NOX-A12 with extracellular GAGs that bind CXCL12 can be considered the mechanism-of-action for NOX-A12–induced mobilization of hematopoietic stem and leukemia cells as previously reported in the clinical phase 1 study of NOX-A12, where a long-lasting increase of hematopoietic cell numbers in the peripheral blood of healthy volunteers was achieved.23 Given the fact that plasma half-life of NOX-A12 is approximately 40 hours,22 CXCL12 action is inhibited for a long period and distinguishes NOX-A12–dependent mobilization from other, more short-term mobilizers such as plerixafor and AMD3100. This feature of longer-term mobilization of leukemia cells makes NOX-A12 an attractive agent for chemosensitization in diseases like CLL. Future dissection in correlative studies in the ongoing first clinical trial of NOX-A12 in CLL24 will help us to better define the effect of NOX-A12 on CLL cell migration and chemosensitization.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors thank Dr Michael Andreeff (MD Anderson Cancer Center, Houston, TX) for providing BMSC samples.
This work was supported by grants from the CLL Global Research Foundation (W.G.W. and J.A.B.), grants from NOXXON, Cancer Prevention and Research Institute of Texas (J.A.B.), and a Leukemia & Lymphoma Society Scholar Award in Clinical Research (J.A.B.).
Authorship
Contribution: J.H. performed the experiments, analyzed the data, and wrote the paper; D.Z. devised and performed the experiments, analyzed the data, designed the figures, and wrote the paper; C.M. devised and performed the experiments, analyzed the data, and wrote the paper; A.K. and N.Y.R. devised the experiments, analyzed the data, and reviewed the manuscript; W.G.W. and M.J.K. provided patient samples and reviewed the manuscript; and J.A.B. designed the research, supervised the study, analyzed the data, and wrote and revised the paper.
Conflict-of-interest disclosure: D.Z., C.M., and A.K. are employees of NOXXON Pharma AG. J.A.B. received research funding from NOXXON. The remaining authors declare no competing financial interests.
Correspondence: Jan A. Burger, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org.
References
Author notes
J.H. and D.Z. contributed equally to this study.