Key Points
Genetic variations at the ORM1 locus and concentrations of the encoded protein associate with thrombin generation.
These findings may guide the development of novel antithrombotic treatments.
Abstract
Thrombin, the major enzyme of the hemostatic system, is involved in biological processes associated with several human diseases. The capacity of a given individual to generate thrombin, called the thrombin generation potential (TGP), can be robustly measured in plasma and was shown to associate with thrombotic disorders. To investigate the genetic architecture underlying the interindividual TGP variability, we conducted a genome-wide association study in 2 discovery samples (N = 1967) phenotyped for 3 TGP biomarkers, the endogenous thrombin potential, the peak height, and the lag time, and replicated the main findings in 2 independent studies (N = 1254). We identified the ORM1 gene, coding for orosomucoid, as a novel locus associated with lag time variability, reflecting the initiation process of thrombin generation with a combined P value of P = 7.1 × 10−15 for the lead single nucleotide polymorphism (SNP) (rs150611042). This SNP was also observed to associate with ORM1 expression in monocytes (P = 8.7 × 10−10) and macrophages (P = 3.2 × 10−3). In vitro functional experiments further demonstrated that supplementing normal plasma with increasing orosomucoid concentrations was associated with impaired thrombin generation. These results pave the way for novel mechanistic pathways and therapeutic perspectives in the etiology of thrombin-related disorders.
Introduction
The enzyme thrombin (also called activated factor II) is a central product of the response to vascular injury, displaying procoagulant, anticoagulant, antifibrinolytic, and cellular effects; the magnitude and timing of these effects are critical to normal hemostasis.
The vast majority of thrombin is generated well after the plasma (or blood) clot formation time, which is the traditional endpoint for the activated partial thromboplastin time and prothrombin time assays.1 These tests do not assess the whole coagulation system and also are insensitive to prothrombotic states.2 Thrombin generation assays have recently gained in popularity and are considered useful to measure “global hemostasis,” that is, capturing the complete dynamics of the coagulant response beyond initial clot formation.3
Patients with high levels of thrombin generation are at risk for thrombotic diseases such as acute ischemic stroke,4 venous thromboembolism (VTE),5-8 and myocardial infarction9 while bleeding events are observed in presence of very low thrombin generation.10 In addition, the role of thrombin generation extends far beyond the sole coagulation system. Several recent findings have emphasized its key impact in atherosclerosis,11 diabetic nephropathy,12,13 and inflammatory diseases such as sepsis,14 Crohn disease,15 and sickle cell disease.16
Altogether these observations clearly emphasize the importance of identifying factors controlling the interindividual variability of thrombin generation. Known environmental and biological determinants of thrombin generation are body mass index, estrogen-based therapies, factor II, fibrinogen, antithrombin, tissue factor pathway inhibitor (TFPI) levels in plasma17-19 while 2 functional genetic variants, both in the F2 gene coding for prothrombin, rs1799963 (G20210A), and rs3136516,20 have been robustly shown to associate with thrombin generation levels. The former is a well-established genetic risk factor for VT21 where the rs1799963-A allele is associated with a 2.5-fold increased risk.22 Conversely, the association of the common rs13136516 with VT risk remains questionable. It has been observed in 2 case-control studies23,24 but was not detected in recent genome-wide association studies (GWAS).25-29 Preliminary works also suggested that the rs3136516-G allele could associate with increased risk of systemic lupus erythematous (SLE).30 If confirmed, this association would add support for the role of thrombin generation into inflammatory diseases.
We hypothesized that additional genetic factors, outside the F2 gene, could modulate the potential of a given individual to generate thrombin. To test this hypothesis, we undertook a GWAS in 2 independent populations totaling 1967 subjects using 1000 Genomes based imputation techniques which allowed us to test 6 652 054 single nucleotide polymorphisms (SNPs) for association with 3 thrombin generation parameters: endogenous thrombin potential (ETP), lag time and peak thrombin generation using the calibrated automated thrombography (CAT) method.31 The main findings were tested for replication in 2 additional independent populations gathering 1339 individuals and functional arguments derived from in silico and in vitro experiments were obtained to support the identified novel association.
Methods
Studied populations
Two independent cohorts with both GWAS data and thrombin generation measurements were used for the discovery stage: MARTHA and the Three-City (3C) Study. The main findings of the meta-analysis of these 2 GWAS datasets were tested for replication in 2 additional independent studies, MARTHA12 and FITENAT. Each individual study was approved by its institutional ethics committee and informed written consent was obtained in accordance with the Declaration of Helsinki. All subjects were of European descent.
Discovery cohorts
The MARTHA study has already been extensively described.32,33 It is composed of 1542 patients with venous thrombosis (VT) recruited from the thrombophilia center of La Timone hospital (Marseille, France). All subjects, with a documented history of VT, were free of any chronic conditions and free of any thrombophilia including anti-thrombin, protein C and protein S deficiencies, and homozygosity for factor V Leiden and factor II G20210A mutations. Patients under anticoagulant therapy were also excluded. The 3C study34 is a population-based study carried out in 3 French cities composed of 8707 noninstitutionalized individuals aged over 65 randomly selected from the electoral rolls and free of any chronic diseases and for which biological (DNA, plasma) samples could have been obtained.
Replication cohorts
The MARTHA12 study is composed of an independent sample of 1245 VT patients that have been recruited between 2010 and 2012 according to the same criteria as the MARTHA patients. The FITENAT sample35 consists of 543 French healthy individuals selected from health examination centers of the French Social Security. These subjects had no history of cardiovascular disease, diabetes, hypertension, renal nor hepatic failure and were not under anticoagulant therapy.
Biological measurements
In all studies, thrombin generation potential (TGP) was measured in platelet-poor plasma (PPP) using the CAT method36 as extensively described in Lavigne-Lissalde et al.18 Three biological TGP parameters were derived from the thrombogram analysis: the ETP (in nmol min−1) which corresponds to the area under the thrombogram curve, the peak thrombin generation (peak in nmol L−1) which represents the maximum amount of thrombin produced after induction by 5pM tissue factor (TF), and the lag time (LagT in minutes) which represents the time to the initial generation of thrombin after induction.
In vitro functional studies
Plasma orosomucoid levels were determined using an automated turbidimetric immunoassay based on the use of a polyclonal rabbit anti-human orosomucoid covalently attached to polystyrene microparticles resulting in a ready to use immunoparticle reagent. All reagents were from Dako A/S and all assays were performed on a Vitros 5.1 from Ortho Clinical Diagnostics.
Orosomucoid (Cell Science) was added at the concentrations of 0.2, 0.4, 0.8, 1.2 and 1.6 mg/mL of plasma to 80 μL of PPP dispensed into the wells of round-bottom 96 well-microtiter plates (Immunolon microtiter 96-well solid plates; Fischer Scientific). These concentrations were chosen for corresponding to the normal range of orosomucoid in plasma (0.6-1.6 mg/mL). Thrombin generation was then initiated by adding 20 μL of PPP reagent (Stago) containing 5 pM TF and 4 µM phospholipid mixture, and measured using the CAT method in a Fluoroskan Ascent fluorometer (Thermolab Systems OY) equipped with a dispenser. Fluorescence intensity was detected at wavelengths of 390 nm (excitation filter) and 460 nm (emission filter). The starting reagent FLUCA Kit (Stago) containing fluorogenic substrate and CaCl2 was automatically dispensed by the fluorometer (20 μL per well). A dedicated software program, Thrombinoscope (Stago) was used to calculate thrombin activity against the Calibrator (Stago) and display thrombin activity vs time. ETP, peak, and lag time were calculated from the thrombogram. These experiments were conducted on normal plasma samples from healthy random individuals, Wilcoxon-paired test statistic was used to assess the association of orosomucoid with TGP biomarkers.
Genotyping and imputation
Plasma were available for TGP measurements in 848 MARTHA patients and 1314 3C subjects with genome-wide genotype data typed with Illumina Human610 and Huma660W Quad beadchips.28 In each study, SNPs with genotyping call rate <99%, minor allele frequency (MAF) <1%, and showing significant (P < 10−5) deviation from the Hardy-Weinberg equilibrium37 were filtered out. This led to 491 285 and 487 154 quality-control (QC) validated autosomal SNPs in MARTHA and 3C, respectively. Individuals were excluded according to the following criteria: genotyping rate <95%, close relatedness as suspected from pairwise clustering of identity by state distances and multidimensional scaling implemented in PLINK,38 and genetic outliers detected by principal components analysis as implemented in the EIGENSTRAT program.39 Finally, 714 and 1253 individuals from the MARTHA and 3C study, respectively, were left for association analyses.
The 467 355 QC-checked SNPs common to both MARTHA and 3C were then used for imputing 11 572 501 autosomal SNPs from the 1000 Genomes 2010-08 release reference dataset. For this, the MACH (version 1.0.18.c) software was used.40 All SNPs with acceptable imputation quality (r2 > 0.3)29,41 and MAF > 0.01 in both imputed GWAS datasets were kept for association analysis.
In the replication cohorts, genotyping was performed using allele-specific PCR in 733 MARTHA12 patients and 528 FITENAT subjects in which TGP measurements and DNA were available.
Statistical analysis
Discovery analysis
In order to handle nonnormality distributions, a log-transformation and a normal quantile transformation42 were applied to ETP and lag time values, respectively, separately in the 2 cohorts (supplemental Figure 1, available on the Blood Web site). Association of imputed SNPs with TGP markers were assessed independently in each cohort by a linear regression model implemented in the MACH2QTL (version 1.1.0)40 software. In this model, the allele dosage, a real number ranging from 0 to 2 and equal to the expected number of minor alleles computed from the posterior probabilities of possible imputed genotypes, is used for assessing the imputed SNP effect. Analyses were adjusted for age, gender, oral contraception therapy (in MARTHA), oral coagulant therapy (3C), and the first 4 principal components. Results obtained in the 2 GWAS cohorts were entered into a fixed-effect meta-analysis relying on the inverse-variance weighted method as implemented in the METAL program.43 Homogeneity of associations across the 2 studies was assessed using the Mantel-Haenszel method.44 A statistical threshold of 5 × 10−8 was used to declare genome-wide significance.41,45,46 In order to increase the sensitivity of our discovery phase, we also considered of potential interest any SNP that did not reach genome-wide significance but were nevertheless associated at P < 10−5 with at least 2 TGP biomarkers.
Conditional analysis
A second round of GWAS analysis was performed where we further conditioned on the Prothrombin G20210A (rs1777963) mutation, a known strong genetic determinants of TGP markers. Because the rs1777963 has been genotyped in the MARTHA study as part of the inclusion criteria, the true genotypes were then used in the conditional MARTHA GWAS while, in the 3C study, the imputed allele dose were used.
Replication analysis
The same transformations as in the discovery cohorts were applied to ETP and lag time values in MARTHA12 and FITENAT. Association of tested SNPs with TGP markers was also assessed by a linear regression model under the assumption of additive allele effects, adjusted for age, sex, and oral contraception therapy.
In silico association with gene expression
The identified ORM1 hit SNP was investigated for association with the expression of its corresponding gene in monocytes and macrophages. Two genome-wide expression studies were used, the Cardiogenics Transcriptomics Study (CTS)47,48 and the Gutenberg Health Study.48,49
For this work, CTS individuals initially typed for the Illumina Sentrix Human Custom 1.2M and human 610-Quad beadchips and GHS subjects typed for Affymetrix Genome-Wide Human SNP Array 6.0 were separately imputed by the MACH (version 1.0.18.c) software according to the 1000 Genomes February 2012 reference database. RNA genome-wide expression from monocytes and macrophages was assessed in CTS using the Illumina HumanRef 8 version 3 Beadchip. In GHS, the Illumina HT-12 version 3 expression array was used to assess monocyte expression. In both datasets, ORM1 gene expression was characterized by the ILMN_1696584 probe.
Association of the hit SNP with gene expression was tested by use of a linear regression model adjusted for age, sex, and center (in CTS).
Results
Characteristics of the studied populations
| . | Discovery . | Replication . | ||
|---|---|---|---|---|
| MARTHA, N = 714 . | 3C, N = 1253 . | MARTHA12, N = 796 . | FITENAT, N = 543 . | |
| Age, y (SE) | 46.84 (15.29) | 75.05 (5.75) | 49.74 (15.34) | 47.81 (13.94) |
| Sex, % male | 32.1 | 41.6 | 42.9 | 47.9 |
| VT patients, % | 100 | 0 | 100 | 0 |
| BMI, kg/m2 (SE) | 25.1 (4.55) | 25.8 (4.22) | 26.0 (4.98) | 24.2 (3.62) |
| FV Leiden (%)* | 153 (21.4) | — | 89 (11.2) | — |
| F2 G20210A (%)* | 83 (11.6) | — | 52 (6.5) | — |
| Oral anticoagulant (%) | — | 52 (4.2) | 24 (3.0) | — |
| BMI, kg/m2 | 25.14 (4.55) | 26.03 (4.98) | ||
| ETP, nM/min (1st-3rd) | 1780 (1554-1958) | 1775 (1586-1947) | 1892 (1654-2124) | 1675 (1456-1838) |
| Peak, nM (1st-3rd) | 333.0 (293.5-372.0) | 332.8 (307.0-364.3) | 328.0 (278.0-374.8) | 293.5 (258.1-319.2) |
| Lag time, min (1st-3rd) | 3.229 (2.830-3.500) | 1.382 (1.000-1.670) | 3.330 (2.762-3.670) | 2.280 (2.000-2.500) |
| . | Discovery . | Replication . | ||
|---|---|---|---|---|
| MARTHA, N = 714 . | 3C, N = 1253 . | MARTHA12, N = 796 . | FITENAT, N = 543 . | |
| Age, y (SE) | 46.84 (15.29) | 75.05 (5.75) | 49.74 (15.34) | 47.81 (13.94) |
| Sex, % male | 32.1 | 41.6 | 42.9 | 47.9 |
| VT patients, % | 100 | 0 | 100 | 0 |
| BMI, kg/m2 (SE) | 25.1 (4.55) | 25.8 (4.22) | 26.0 (4.98) | 24.2 (3.62) |
| FV Leiden (%)* | 153 (21.4) | — | 89 (11.2) | — |
| F2 G20210A (%)* | 83 (11.6) | — | 52 (6.5) | — |
| Oral anticoagulant (%) | — | 52 (4.2) | 24 (3.0) | — |
| BMI, kg/m2 | 25.14 (4.55) | 26.03 (4.98) | ||
| ETP, nM/min (1st-3rd) | 1780 (1554-1958) | 1775 (1586-1947) | 1892 (1654-2124) | 1675 (1456-1838) |
| Peak, nM (1st-3rd) | 333.0 (293.5-372.0) | 332.8 (307.0-364.3) | 328.0 (278.0-374.8) | 293.5 (258.1-319.2) |
| Lag time, min (1st-3rd) | 3.229 (2.830-3.500) | 1.382 (1.000-1.670) | 3.330 (2.762-3.670) | 2.280 (2.000-2.500) |
BMI, body mass index; FV, factor V.
FV Leiden and F2 G20210A mutations were genotyped in MARTHA and MARTHA12 as part of the inclusion criteria. Homozygous carriers were not included in the study.
Correlation between TGP markers
| . | MARTHA . | 3C . | MARTHA12 . | FITENAT . |
|---|---|---|---|---|
| ETP − Peak, r | 0.78 | 0.77 | 0.73 | 0.78 |
| ETP − Lag time, r | 0.14 | 0.06 | 0.19 | 0.34 |
| Peak − Lag time, r | −0.05 | −0.10 | −0.18 | 0.04 |
| . | MARTHA . | 3C . | MARTHA12 . | FITENAT . |
|---|---|---|---|---|
| ETP − Peak, r | 0.78 | 0.77 | 0.73 | 0.78 |
| ETP − Lag time, r | 0.14 | 0.06 | 0.19 | 0.34 |
| Peak − Lag time, r | −0.05 | −0.10 | −0.18 | 0.04 |
Correlations were computed on transformed values (ie, log-transformation on ETP and quantile normalization for lag time) adjusted for age, sex, oral anticoagulant therapy, F2 G20210A (when appropriate), and BMI.
Abbreviations are explained in Table 1.
Discovery meta-analysis
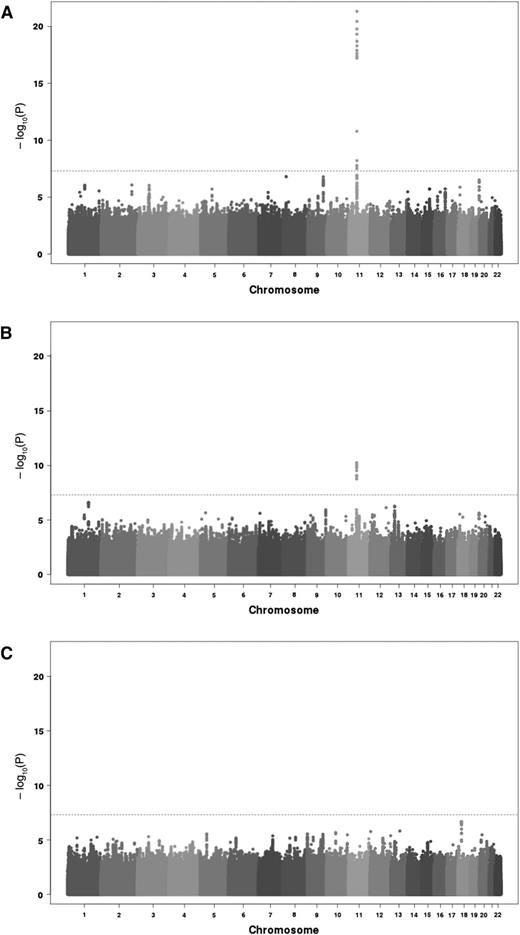
A total of 6 652 054 imputed SNPs common to both GWAS cohorts satisfied pre-specified imputation quality criteria and were then tested through meta-analysis for association with the 3 TGP phenotypes, ETP, peak, and lag time. Quantile-quantile (Q-Q) plots of the association results did not reveal any inflation from what expected under the null hypothesis of no association, except for the extreme right tail distribution for ETP and peak (supplemental Figure 2) Corresponding genomic inflation coefficients were 0.998, 0.997, and 1.000 for ETP, peak, and lag time, respectively. Manhattan plot representation of the results are depicted in Figure 1.
Manhattan plots of the association results from the meta-analysis of 2 discovery cohorts imputed for 6 652 054 SNPs on 3 TGP biomarkers. ETP (A), peak (B), and lag time (C). The horizontal line corresponds to the genome-wide significant threshold taken at 5 × 10−8.
Manhattan plots of the association results from the meta-analysis of 2 discovery cohorts imputed for 6 652 054 SNPs on 3 TGP biomarkers. ETP (A), peak (B), and lag time (C). The horizontal line corresponds to the genome-wide significant threshold taken at 5 × 10−8.
ETP analysis
Seventeen SNPs, all mapping the chromosome 11p11 region, reached genome-wide significance for association with ETP (supplemental Table 1). The strongest signal was observed for MYBPC3 rs2856656 (P = 4.62 × 10−22). As we have previously shown that this SNP tags for the F2 G20210A (rs1799963) mutation,29 a further GWAS meta-analysis was conducted by conditioning on the rs1799963. The imputation quality criteria of the rs1799963 was r2 = 1 in MARTHA (see “Methods”) and r2 = 0.274 in 3C. The rare rs1799963-A allele was much more frequent in MARTHA thus allowing better imputation. As a consequence, it was not included in the initial set of imputed SNPs that entered the GWAS analysis. After adjusting for rs1799963, 14 SNPs remained significantly associated with ETP. The strongest association was observed for the F2 rs3136516 (P = 5.94 × 10−14). After a further round of adjustment on the rs3136516 allele dosage, no association remained genome-wide significant. A 2-locus model incorporating the rs1799963 and rs3136516 revealed that their effects on ETP were independent and highly significant (P = 1.02 × 10−29). The rs1799963-A (β = +0.225 ± 0.019, P = 2.66 × 10−31) and the rs3136516-G (β = +0.040 ± 0.006, P = 5.89 × 10−11) alleles were both associated with increased ETP. These effects were homogeneous in MARTHA (β = +0.224 ± 0.019; β = +0.042 ± 0.009, respectively) and in 3C (β = +0.287 ± 0.135, β = +0.039 ± 0.008, respectively), with no statistical difference (P > .05) between studies. It is worthy of note that, while the rs1799963-A allele was much more frequent in MARTHA patients than in 3C healthy subjects (0.058 vs 0.005), there was no difference in the rs3136516-G allele frequencies (0.48 vs 0.47, respectively)
Peak analysis
Twelve SNPs at the 11p11.2 locus were significantly associated with peak, the strongest signal being for the rs138315285 (P = 5.48 × 10−11) and the second hit being the rs2856656 (P = 8.29 × 10−11) (supplemental Table 2). After adjusting for rs1799963, 2 associations, rs3136512 and rs3136516, remained statistically significant (both P = 2.91 × 10−8). These 2 SNPs were in perfect linkage disequilibrium (LD). In a joint model, the rs1799963-A and rs3136516-G alleles were independently associated with increased peak levels, β = +45.37 ± 7.45 (P = 1.10 × 10−9) and β = +9.79 ± 2.03 (P = 1.36 × 10−6), respectively. These effects were of similar amplitude in MARTHA (β = +44.37 ± 7.58; β = +12.26 ± 3.56, respectively) and in 3C (β = +73.22 ± 39.97; β = +8.62 ± 2.46, respectively).
Lag time analysis
No SNP was genome-wide significantly associated with lag time. Of note, the F2 rs1799963 and rs3136516 did not associate with lag time (P = .096 and P = .451, respectively).
“Joint analysis” of TGP phenotypes
As the GWAS analyses of TGP phenotypes did not reveal any genome-wide significant association signal independent of the known F2 variants (rs1799963 and rs3136516), we followed up additional SNPs that demonstrated suggestive evidence for association (P < 10−5) with at least 2 of the 3 studied TGP biomarkers.
Four SNPs, all mapping to the chromosome 9 ORM1 gene, demonstrated suggestive evidence for association with both ETP and lag time (supplemental Table 3). The strongest association was observed for rs150611042 whose A rare allele was associated with lower ETP (β = −0.068 ± 0.013, P = 3.36 × 10−7) and lag time (β = −0.338 ± 0.073, P = 4.10 × 10−7) with no evidence for heterogeneity between MARTHA and 3C (P = .729 and P = .912, respectively). After adjusting for rs150611042, the association at the other ORM1 SNPs completely vanished confirming that these 4 SNPs were in strong LD as initially anticipated from the similarity in their allele frequencies and associated genetic effects.
Thirty-eight SNPs were suggestively associated with both ETP and peak, most of them being located in the 11p11 region discussed above. After adjusting for rs1799963 and rs3136516 F2 variants, we observed suggestive association between a block of 7 SNPs mapping to the RPL7AP69 locus on chromosome 19q13.43 and ETP and peak (supplemental Table 4). All these SNPs were in nearly complete association, the minor allele of the best associated SNP (rs117368154) was associated with decreased ETP (β = −16.29 ± 3.48, P = 2.83 × 10−6) and peak (β = −0.054 ± 0.011, P = 3.39 × 10−7).
No SNP exhibited association at P < 10−5 with both peak and lag time.
Replication studies
Following the main findings derived from the discovery meta-analysis, ORM1 and RPL7AP69 hit SNPs were tested for association with TGP phenotypes in MARTHA12. No association with any TGP biomarker was observed for the RPL7AP69 SNP (supplemental Table 5). Conversely, the ORM1 rs150611042 demonstrated significant association (P = 2.46 × 10−7) with lag time but not with ETP (P = .147). Consistent with the discovery results, the rs150611042-A allele was associated with decreased lag time (β = −0.439 ± 0.084). To provide additional support for this association, the rs150611042 was genotyped in the FITENAT study where its A allele was also associated with decreased lag time (β = −0.280 ± 0.099, P = 5.04 × 10−3) (Table 3).
Association of ORM1 rs150611042 with biomarkers of thrombin generation in 4 independent studies
| ORM1 rs150611042 C/A . | MARTHA, N = 714 . | 3C, N = 1253 . | MARTHA12, N = 726 . | FITENAT, N = 528 . | Combined,* N = 3221 . |
|---|---|---|---|---|---|
| Minor allele frequencies | 0.089 | 0.082 | 0.096 | 0.095 | |
| Lag time | |||||
| β† (SE) | −0.329 (0.086) | −0.343 (0.096) | −0.439 (0.084) | −0.280 (0.099) | −0.354 (0.045) |
| P | 1.53 × 10−4 | 3.85 × 10−4 | 2.46 × 10−7 | 5.04 × 10−3 | 7.11 × 10−15 |
| ETP | |||||
| β (SE) | −0.041 (0.016) | −0.083 (0.018) | −0.024 (0.017) | −0.013 (0.017) | −0.038 (0.009) |
| P | .015 | 9.31 × 10−6 | .147 | .449 | 8.41 × 10−6 |
| Peak | |||||
| β (SE) | −8.379 (6.107) | −6.557 (5.516) | 8.730 (6.286) | −0.984 (4.592) | −2.013 (2.748) |
| P | .171 | .233 | .165 | .830 | .464 |
| ORM1 rs150611042 C/A . | MARTHA, N = 714 . | 3C, N = 1253 . | MARTHA12, N = 726 . | FITENAT, N = 528 . | Combined,* N = 3221 . |
|---|---|---|---|---|---|
| Minor allele frequencies | 0.089 | 0.082 | 0.096 | 0.095 | |
| Lag time | |||||
| β† (SE) | −0.329 (0.086) | −0.343 (0.096) | −0.439 (0.084) | −0.280 (0.099) | −0.354 (0.045) |
| P | 1.53 × 10−4 | 3.85 × 10−4 | 2.46 × 10−7 | 5.04 × 10−3 | 7.11 × 10−15 |
| ETP | |||||
| β (SE) | −0.041 (0.016) | −0.083 (0.018) | −0.024 (0.017) | −0.013 (0.017) | −0.038 (0.009) |
| P | .015 | 9.31 × 10−6 | .147 | .449 | 8.41 × 10−6 |
| Peak | |||||
| β (SE) | −8.379 (6.107) | −6.557 (5.516) | 8.730 (6.286) | −0.984 (4.592) | −2.013 (2.748) |
| P | .171 | .233 | .165 | .830 | .464 |
Abbreviations are explained in Table 1.
Combined results were derived from a meta-analysis of the 4 studies under the framework of an inverse-variance weighting fixed-effect model. No heterogeneity was observed across cohorts, I2 = 1.67 (P = .795), I2 = 8.59 (P = 0.072), and I2 = 4.74 (P = .314) for lag time, ETP, and peak, respectively.
Additive effects associated with the rs150611042-A allele, adjusted for age, sex, oral contraceptive therapy (except in 3C), and on first 4 principal components (in MARTHA and 3C). In 3C, as well in 25 MARTHA patients, imputed allele dosage was used. Otherwise, the exact allele count derived from wet-laboratory genotyping was used. Association was tested by use of a linear regression model.
rs150611042 was imputed in the discovery GWAS. As a consequence, we de novo genotyped it in 689 patients of the MARTHA GWAS where DNA was still available. In this sample, the Pearson correlation between the imputed dose and the true genotype was ρ = 0.73. The association of rs150611042 with lag time was slightly stronger using true genotyped allele count (β = −0.338 ± 0.087, P = 1.11 × 10−4) than that observed using imputed allele dose (β = −0.362 ± 0.127, P = 4.71 × 10−3).
Finally, the meta-analysis of the 4 studies provide strong statistical evidence for the association of rs150611042 with lag time (P = 7.11 × 10−15) with no evidence for heterogeneity across studies (P = .795) (Table 3). In the combined samples totaling 3,221 subjects, the decreasing effect of the rs150611042-A allele was β = −0.354 ± 0.045. No evidence for heterogeneity according to the presence of the rs3136516-G allele was observed (P = .172). Similarly, in the 2 combined VT samples enriched for F2 G20210A mutation, the rs150611042-A effect on lag time was homogeneous (test for homogeneity P = .746) in patients with (β = −0.300 ± 0.255) or without (β = −0.385 ± 0.062) the rs1799963-A allele. Further adjustment of BMI did not alter the detected association (β = −0.343 ± 0.044, P = 1.08 × 10−14)
No association of rs150611042 with peak nor ETP was observed (Table 3).
In silico association with gene expression
In the CTS and GHS studies, the rs150611042 was correctly imputed, with r2 = 0.64 and r2 = 0.75, respectively. In both studies, the rs150611042-A allele was significantly (P = 8.70 × 10−10 and P = 5.21 × 10−16 in CTS and GHS, respectively) associated with decreased expression in monocytes in an additive manner (Table 4) and explained ∼5% of the variability of ORM1 monocyte gene expression. A similar pattern of association, although less significant (P = 3.18 × 10−3), was observed for ORM1 gene expression in macrophages from CTS (Table 4).
Association of ORM1 rs150611042 with ORM1 monocyte and macrophage expression
| ORM1 rs150611042 . | CTS . | GHS . | |||
|---|---|---|---|---|---|
| N . | Monocyte . | Macrophage . | N . | Monocyte . | |
| CC* | 664 | 6.80 (0.57) | 6.19 (0.21) | 1175 | 6.81 (0.38) |
| CA* | 79 | 6.45 (0.48) | 6.12 (0.11) | 196 | 6.62 (0.26) |
| AA* | 2 | 5.99 (0.09) | 6.10 (0.09) | 3 | 6.36 (0.04) |
| R2, % | 4.80 | 1.30 | 4.90 | ||
| P† | 8.70 × 10−10 | 3.18 × 10−3 | 5.21 × 10−16 | ||
| ORM1 rs150611042 . | CTS . | GHS . | |||
|---|---|---|---|---|---|
| N . | Monocyte . | Macrophage . | N . | Monocyte . | |
| CC* | 664 | 6.80 (0.57) | 6.19 (0.21) | 1175 | 6.81 (0.38) |
| CA* | 79 | 6.45 (0.48) | 6.12 (0.11) | 196 | 6.62 (0.26) |
| AA* | 2 | 5.99 (0.09) | 6.10 (0.09) | 3 | 6.36 (0.04) |
| R2, % | 4.80 | 1.30 | 4.90 | ||
| P† | 8.70 × 10−10 | 3.18 × 10−3 | 5.21 × 10−16 | ||
CTS, Cardiogenics Transcriptomics Study; GHS, Gutenberg Health Study.
Mean (SD).
Association testing was performed by regressing ORM1 expression on the imputed allele dosage of rs150611042 while adjusting for age, sex, and center (in CTS).
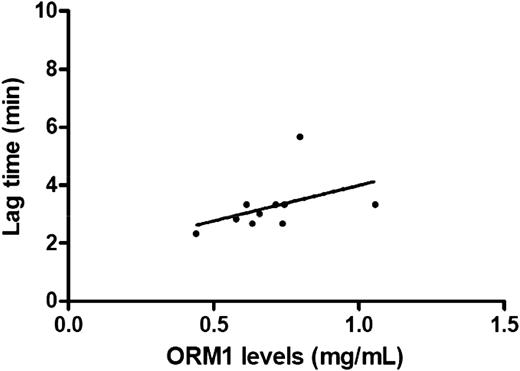
In vitro functional studies
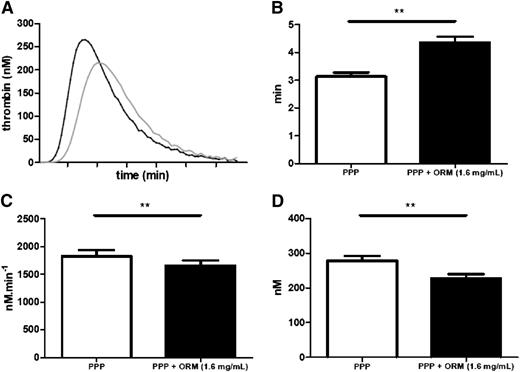
As illustrated in Figure 2, orosomucoid levels were positively correlated (r2 = 0.646, P = .049) with lag time in a plasma sample of 10 individuals. As shown in supplemental Figure 3, there was a positive dose-dependent association between orosomucoid concentrations and lag time. For instance, at the 1.6 mg/mL concentration, the supplementation of orosomucoid to PPP was followed by a modification of the thrombin activity characterized by significant increased lag time (3.25 vs 4.50 miutesn, P = .0057) but also significant decreased ETP (1790 vs 1628 nmol L−1, P = .0020) and peak (266 vs 213 nmol L−1P = .002) (Figure 3).
Association of lag time with plasma ORM1 levels. In 10 normal plasma, the correlation between ORM1 and lag time was 0.646 (P = .049).
Association of lag time with plasma ORM1 levels. In 10 normal plasma, the correlation between ORM1 and lag time was 0.646 (P = .049).
Influence of orosomucoid supplementation on thrombin generation. (A) Thrombin generation curves from 10 normal plasma that were (gray curves) or were not supplemented (black curves) with 1.6 mg/mL orosomucoid. Effect of supplementation of PPP with 1.6 mg/mL orosomucoid over lag time (B), ETP (C), and peak (D). Measurements were performed in duplicates and the mean values were used for each individual. **P < .01.
Influence of orosomucoid supplementation on thrombin generation. (A) Thrombin generation curves from 10 normal plasma that were (gray curves) or were not supplemented (black curves) with 1.6 mg/mL orosomucoid. Effect of supplementation of PPP with 1.6 mg/mL orosomucoid over lag time (B), ETP (C), and peak (D). Measurements were performed in duplicates and the mean values were used for each individual. **P < .01.
Discussion
Here, we reported the results of a GWAS study aimed at identifying genetic variations associated with thrombin generation through a comprehensive analysis of 3 complementary biomarkers, ETP, peak and lag time.
For ETP and peak, associations were observed with rs1799963 and rs3136516, 2 F2 variants already known to associate with thrombin generation and whose functionality has already been discussed.20,50-54 Both rare alleles, rs1799963-A and rs3136516-G, were associated independently from each other with increased thrombin generation, the strongest effect being at rs1799963. These 2 mutations act on thrombin generation through increase in prothrombin levels. The mechanism by which the rs1799963-A, located in the 3′ UTR region of F2, influences prothrombin levels has been proposed to result from more efficient 3′-end formation, increased mRNA stability, increased translation efficiency, or a combination of these mechanisms.50,54 The rs3136516-G, located within the 13th intron of the gene, is functional through its effect on an intronic splicing enhancer motif.20 Of note, while the rs1799963-A allele is a rare (∼2% in the general population) variant associated with a strong risk of VT, the rs3136516-G allele is common (∼0.47 in all populations studied here) and its association with VT risk still warrants in-depth investigation.
The novelty of this work is the association of ORM1 rs150611042-A allele with decreased lag time in 4 independent studies, with an overall statistical evidence of P = 7.11 × 10−15. Although this polymorphism explained ∼2% of the lag time variability (1%, 1.7%, 3.4%, and 1.4% in MARTHA, 3C, MARTHA12 and FITENAT, respectively), it was not associated with either ETP or peak. No evidence for association with VT risk was suggested either, the frequency of the rs150611042-A allele (Table 3) being homogeneous across the 2 cohorts of VT patients (0.089 and 0.096) and the 2 cohorts of healthy individuals (0.082 and 0.095). The rs150611042 is located in the promoter region of the ORM1 gene and was in strong LD with other ORM1 SNPs whose association with lag time disappeared after adjusting for rs150611042. Using transcriptomic data, we further observed that the rs150611042-A allele was associated with decreased ORM1 expression in monocytes and macrophages. Finally, in vitro functional studies revealed that plasma orosomucoid levels correlate with lag time and that supplementing the plasma of healthy individuals with orosomucoid resulted in impaired thrombin activity as characterized by increased lag time; this observation being consistent with the concomitant associations of rs150611042-A allele with both decreased lag time and decreased ORM1 expression.
ORM1 encodes a key acute phase plasma protein, orosomucoid also called α-1-acid glycoprotein 1 (α-1-AGP)55 whose specific function has not yet been determined; it might function as a transport protein in the blood stream, appears to modulate the immune system during the acute-phase reaction and has been shown to associate with allergic contact dermatitis, psoriasis, and sarcoidosis.56 Several pieces of evidence support a role of α-1-AGP in coagulation. In an experimental model of peritonitis in rats, high doses of α-1-AGP normalized platelet aggregation, blood clotting parameters and antithrombin activity.57 Increased amounts of α-1-AGP inhibit platelet aggregation induced by ADP and adrenaline.58 α-1-AGP was also found to interact with plasminogen activator inhibitor 1, a member of the serine proteinase inhibitors (serpins) family, and to stabilize its inhibitory activity toward plasminogen activators.59 One could speculate that α-1-AGP delays thrombin generation by playing the same role with other coagulation inhibitors belonging to the serpin family. Conversely, α-1-AGP has been observed to shorten aPTT60 and to contribute to the cellular initiation of coagulation by inducing monocyte expression of TF.61
Our study provides evidence of the role of α-1-AGP in thrombin generation, in particularly on the initiation process evaluated through the lag time biomarker. Several limitations must be acknowledged. First, the 2 discovery cohorts as well as the 2 replication studies were composed of samples of VT patients and healthy individuals exhibiting different clinical and biological characteristics. This may then have introduced heterogeneity between studied individuals resulting in a decreased power for detecting genetic effects, in particular of modest effect size. Conversely, the association of ORM1 SNPs with lag time observed in these 4 different cohorts is a strong argument in favor of a true association. The ORM1 locus was selected for further investigation in the replication studies because of its suggestive association (P < 10−5) with both ETP and lag time in the combined discovery cohorts. However, only the association with lag time robustly replicated. As the correlation between ETP and lag time was stronger in the replication studies than in the discovery cohorts, we could have anticipated that the association with ETP would also hold in the replication. Therefore, we cannot rule out that the original association with ETP was spurious. We observed strong statistical evidence for association of our hit SNP with ORM1 expression in monocytes and macrophages, but its influence on gene expression in other cell types would also be of great interest, in particular in hepatocytes, the main source of orosomucoid. Finally, while our in vitro experiments confirmed the association of ORM1 with lag time, they also strongly suggested some associations with both ETP and peak biomarkers which were not robustly suspected from the GWAS investigations. This discrepancy could be explained by the fact that the identified SNP explains a modest part of the ORM1 variability (eg, ∼5% in monocytes) which could be only enough to detect an influence on lag time, whereas our in vitro experiments were able to reflect a more global and stronger effect of orosomucoid on all TGP biomarkers. This would emphasize the need for an in-depth investigation of all genetic and non-genetic (incl. epigenetic) factors influencing ORM1 expression and their impact on thrombin generation. In particular, based on our preliminary results, it would be highly relevant to assess in larger populations the correlation between plasma ORM1 and TGP biomarkers and whether this correlation could be influenced by ORM1 SNPs.
Despite these limitations, our results strongly support a role of ORM1 in thrombin generation-related mechanisms. The impact of the identified SNP on thrombin generation is mild and does not seem to be sufficient to modify the risk of VT in the general population. Orosomucoid is an acute phase reaction protein which increases in concentration as much as 5-fold in acute inflammation and cancer.59 We can speculate that its direct influence on thrombin generation might be higher during inflammation process and thus responsible for coagulation disorders observed in such circumstances. Lastly, the association of the identified ORM1 polymorphism with thrombin-associated human diseases (eg, Crohn, SLE, diabetic nephropathy, stroke) would warrant further investigations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
A.R.-A. was supported by a grant from the Regional Ile de France (CORDDIM). The MARTHA project was supported by a grant from the Program Hospitalier de Recherche Clinique and a grant from CSL Behring. The 3C Study is conducted under a partnership agreement between Inserm, the Victor Segalen–Bordeaux II University and Sanofi-Synthélabo. The Fondation pour la Recherche Médicale funded the preparation and first phase of the study. The 3C Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Mutuelle Générale de l’Education Nationale, the Institut de la Longévité, Agence Française de Sécurité Sanitaire des Produits de Santé, the Regional Governments of Aquitaine, Bourgogne and Languedoc-Roussillon, and the Fondation de France, the Ministry of Research-Inserm Programme “Cohorts and collection of biological material.” The Lille Génopôle received an unconditional grant from Eisai. The 3C Study was supported by a grant from the Agence Nationale pour la Recherche (ANR 2007-LVIE-005-01). CARDIOGENICS was funded by the European Union FP6 program (LSHM-CT-2006-037593). Collection of the Cardiogenics controls was in part supported through the Cambridge Bioresource, which is funded by the NIHR Cambridge Biomedical Research Centre. The Gutenberg Health Study is funded through the government of Rheinland-Pfalz (‘‘Stiftung Rheinland Pfalz für Innovation,’’ contract AZ 961-386261/733), the research programs ‘‘Wissenschafft Zukunft’’ and ‘‘Schwerpunkt Vaskulare Prävention’’ of the Johannes Gutenberg-University of Mainz and its contract with Boehringer Ingelheim and PHILIPS Medical Systems, including an unrestricted grant for the Gutenberg Health Study. This study was supported by the National Genome Network ‘‘NGFNplus’’ (contract A3 01GS0833 and 01GS0831) and by joint funding from the Federal Ministry of Education and Research, Germany (contract BMBF 01KU0908A), and from the Agence Nationale de la Recherche, France (contract ANR 09 GENO 106 01), for the project CARDomics. P.S.W. is funded by the Federal Ministry of Education and Research (BMBF 01EO1003).
Statistical analyses used the C2BIG Computing Centre funded by the Regional Ile de France (“CORRDIM”) and the Pierre and Marie Curie University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: A.R.-A. performed all statistical analyses in the discovery and replication studies, and drafted the manuscript; W.C. contributed to patient and biological data collection; L.C., M.A.-G., M.-C.A., L.L., A.-M.D., M.B., P.A., and P.-Y.S. collected data from the discovery studies; C.F. and N.S. organized the wet-laboratory experiments, including genotyping and in vitro experiments; P.S.W., T.Z., F.C., and A.H.G. coordinated the expression analyses; M.G. and D.-A.T. supervised all statistical works; L.C., C.F., F.C., A.H.G., P.A., P.-Y.S., D.-A.T., and P.-E.M. wrote the manuscript; and D.-A.T. and P.-E.M. designed the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the CardioGenics Consortium appears in “Appendix.”
Correspondence: David-Alexandre Tregouet, INSERM UMR_S 1166, 91 Boulevard de l’Hopital, 75013 Paris, France; e-mail: david.tregouet@upmc.fr; and Pierre-Emmanuel Morange, Laboratory of Haematology, CHU Timone, 246, rue Saint-Pierre, 13385 Marseille Cedex 05, France; e-mail: pierre.morange@ap-hm.fr.
References
Author notes
D.-A.T. and P.-E.M. contributed equally to this work.