Key Points
MGUS patients have significantly increased cortical bone porosity and reduced bone strength relative to matched controls.
Abstract
Patients with monoclonal gammopathy of undetermined significance (MGUS) are at increased fracture risk, and we have previously shown that MGUS patients have altered trabecular bone microarchitecture compared with controls. However, there are no data on whether the porosity of cortical bone, which may play a greater role in bone strength and the occurrence of fractures, is increased in MGUS. Thus, we studied cortical porosity and bone strength (apparent modulus) using high-resolution peripheral quantitative computed tomography imaging of the distal radius in 50 MGUS patients and 100 age-, gender-, and body mass index–matched controls. Compared with controls, MGUS patients had both significantly higher cortical porosity (+16.8%; P < .05) and lower apparent modulus (–8.9%; P < .05). Despite their larger radial bone size, MGUS patients have significantly increased cortical bone porosity and reduced bone strength relative to controls. This increased cortical porosity may explain the increased fracture risk seen in MGUS patients.
Introduction
Population-based studies have shown that fracture risk is increased in monoclonal gammopathy of undetermined significance (MGUS).1,2 Previously,3 we used high-resolution peripheral quantitative computed tomography (HRpQCT) imaging of the distal radius to demonstrate that MGUS patients have significantly altered trabecular bone microarchitecture, but also greater bone size relative to matched controls. However, the impact of these skeletal alterations on bone strength, which can be assessed from HRpQCT images using micro-finite element (µFE) analysis,4 is unknown. Moreover, evidence suggests cortical bone porosity may be more important for bone strength and fracture risk than trabecular bone microarchitectural changes.5,6
Recent work demonstrates that the default HRpQCT cortical bone analysis previously used3 performs poorly for subjects with thin or porous cortices.7 Recognizing this limitation, Burghardt et al8 developed a novel image processing protocol that automatically segments and quantifies cortical bone microarchitecture from HRpQCT images. This permits detection of intracortical pore space morphologically and provides a cortical porosity index shown to increase with age in men and women,5 enhancing identification of subjects at increased fracture risk.6 Notably, so far, this technique has not been applied to patients with any hematologic condition.
Therefore, we used novel advancements in HRpQCT image processing and µFE analysis to determine whether MGUS patients have altered cortical bone microarchitecture and deficits in biomechanical bone strength compared with matched controls.
Study design
Subjects
After Mayo Clinic Institutional Review Board approval, subjects were recruited as previously described3 and written informed consent was obtained in accordance with the Declaration of Helsinki. Subjects included 50 patients diagnosed with MGUS according to International Myeloma Working Group criteria9 and 100 age-, gender-, and body mass index (BMI)-matched (1:2 ratio) controls from an age-stratified random sample of residents in Olmsted County, MN.10 Reflecting the local ethnic composition,11 97% of subjects were white.
Protocol
All procedures were conducted at the Mayo Clinic outpatient Clinical Research Unit (Rochester, MN). Anthropometric data were collected on all subjects. Bone microarchitecture and strength of the nondominant distal radius were assessed by HRpQCT; data from 3 scans (1 MGUS and 2 controls) were excluded because of motion artifact.
HRpQCT imaging
Details regarding distal radial HRpQCT measurements and the default image analysis protocol have been previously described.3 In the present analysis, we used the recently developed extended cortical analysis8 to obtain cortical volumetric bone mineral density (vBMD, mg/cm3), cortical thickness (mm), cortical pore volume (mm3), and cortical porosity (%).
µFE analysis
Linear µFE models were created directly from the HRpQCT images (µFE element analysis solver v.1.15, Scanco Medical AG, Brüttisellen, Switzerland) as previously described.12 Biomechanical bone strength estimates (ie, stiffness, failure load, apparent modulus) were derived from a uniaxial compression test simulating 1% compression, such that 2% of all elements had an effective strain >7000 microstrain. This test simulates a fall from standing height on the outstretched hand, trauma classically associated with Colles’ fractures.13 Failure loads calculated from such µFE models correlate highly (r = 0.87) with compressive loads producing Colles’ fractures in cadaveric forearms.4
Statistical analysis
Comparisons of bone parameters between MGUS patients and controls were made using an analysis of variance model adjusted for age and gender. Testing was performed at a significance level of P < .05 (two-tailed).
Results and discussion
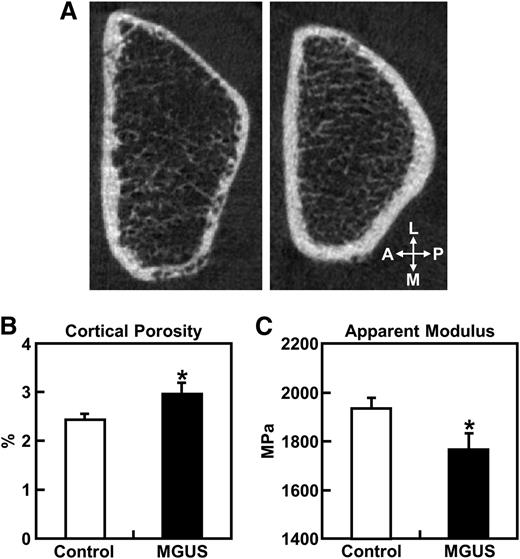
Figure 1A shows representative cross-sectional distal radius HRpQCT images (slice 55 of 110) from a female MGUS patient (left) and an age-, gender-, and BMI-matched control subject (right), with the MGUS patient having both cortical thinning and deficits in cortical vBMD relative to the matched control. Further, MGUS was associated with higher cortical porosity, particularly along the medial and posterior borders of the distal radius.
Radial cortical porosity and bone strength. (A) Representative cross-sectional HRpQCT images of the distal radius (slice 55 of 110) in a female MGUS patient (left panel) and an age-, gender-, and BMI-matched control subject (right panel). (B) Cortical porosity and (C) apparent modulus (bone strength corrected for cross-sectional area) at the distal radius in MGUS patients and controls. Data are shown as mean ± SE adjusted for age and gender. *P < .05 for difference between groups. A, anterior; L, lateral; M, medial; MPa, megapascal; P, posterior; SE, standard error.
Radial cortical porosity and bone strength. (A) Representative cross-sectional HRpQCT images of the distal radius (slice 55 of 110) in a female MGUS patient (left panel) and an age-, gender-, and BMI-matched control subject (right panel). (B) Cortical porosity and (C) apparent modulus (bone strength corrected for cross-sectional area) at the distal radius in MGUS patients and controls. Data are shown as mean ± SE adjusted for age and gender. *P < .05 for difference between groups. A, anterior; L, lateral; M, medial; MPa, megapascal; P, posterior; SE, standard error.
Clinical characteristics and bone parameters of the MGUS and control groups (Table 1) demonstrates that the MGUS and control groups were similar in age, height, weight, and BMI. MGUS patients had significantly higher cortical porosity (+16.8%; P < .05) (Figure 1B), tended to have higher cortical pore volumes (+15.5%; P = .087), and had significant deficits in cortical vBMD (–4.5%; P < .001) vs controls. Further, cortical thickness was lower (–6.6%) in MGUS patients, although this difference only approached significance (P = .067). Biomechanical bone strength parameters (failure load, stiffness, and apparent modulus) were lower in MGUS patients (by –4.0%, –4.6%, and –8.9%, respectively), although only the apparent modulus difference was statistically significant (P < .05) (Figure 1C). Box plots showing the radial bone parameters in the MGUS and control groups are provided in supplemental Figure 1, available at the Blood Web site.
Clinical characteristics and distal radius bone parameters (derived using the extended cortical bone analysis and µFEA of HRpQCT images) in MGUS patients and matched control subjects
| . | MGUS (n = 50) . | Control (n = 100) . | P value . |
|---|---|---|---|
| Clinical characteristics | |||
| Male, n (%) | 30 (60%) | 60 (60%) | |
| Age (y) | 70.5 ± 1.4 | 70.3 ± 1.0 | .878 |
| Height (cm) | 171 ± 1.5 | 170 ± 0.9 | .763 |
| Weight (kg) | 82.2 ± 2.3 | 83.0 ± 1.6 | .789 |
| BMI (kg/m2) | 28.2 ± 0.7 | 28.6 ± 0.4 | .622 |
| Cortical bone parameters (derived by HRpQCT) | |||
| Cortical porosity (%) | 2.91 ± 0.19 | 2.46 ± 0.13 | .048 |
| Cortical pore volume (mm3) | 17.3 ± 1.2 | 14.8 ± 0.8 | .087 |
| Cortical vBMD (mg/cm3) | 907 ± 8.0 | 949 ± 5.7 | <.001 |
| Cortical thickness (mm) | 0.990 ± 0.030 | 1.058 ± 0.021 | .067 |
| Biomechanical bone strength (derived by µFEA) | |||
| Failure load (N) | 4049 ± 112 | 4215 ± 79 | .230 |
| Stiffness (kN/mm) | 80 ± 2.3 | 84 ± 1.6 | .189 |
| Apparent modulus (MPa) | 1768 ± 67 | 1933 ± 47 | .045 |
| . | MGUS (n = 50) . | Control (n = 100) . | P value . |
|---|---|---|---|
| Clinical characteristics | |||
| Male, n (%) | 30 (60%) | 60 (60%) | |
| Age (y) | 70.5 ± 1.4 | 70.3 ± 1.0 | .878 |
| Height (cm) | 171 ± 1.5 | 170 ± 0.9 | .763 |
| Weight (kg) | 82.2 ± 2.3 | 83.0 ± 1.6 | .789 |
| BMI (kg/m2) | 28.2 ± 0.7 | 28.6 ± 0.4 | .622 |
| Cortical bone parameters (derived by HRpQCT) | |||
| Cortical porosity (%) | 2.91 ± 0.19 | 2.46 ± 0.13 | .048 |
| Cortical pore volume (mm3) | 17.3 ± 1.2 | 14.8 ± 0.8 | .087 |
| Cortical vBMD (mg/cm3) | 907 ± 8.0 | 949 ± 5.7 | <.001 |
| Cortical thickness (mm) | 0.990 ± 0.030 | 1.058 ± 0.021 | .067 |
| Biomechanical bone strength (derived by µFEA) | |||
| Failure load (N) | 4049 ± 112 | 4215 ± 79 | .230 |
| Stiffness (kN/mm) | 80 ± 2.3 | 84 ± 1.6 | .189 |
| Apparent modulus (MPa) | 1768 ± 67 | 1933 ± 47 | .045 |
Values are presented as percentage or mean ± SE and P values. Comparisons of bone parameters are adjusted for age and gender.
MPa, megapascal; SE, standard error.
Although MGUS patients are at increased risk for fracture1,2 and progression to multiple myeloma or a related plasma cell cancer,14 clinically, MGUS patients are followed without treatment until progression.15 In this study, we used HRpQCT and µFE analysis to show that MGUS patients have altered cortical microarchitecture and lower biomechanical bone strength vs matched controls, factors that are likely significant for explaining their increased fracture risk.
Although dual-energy X-ray absorptiometry is clinically used for monitoring skeletal health, it cannot separate trabecular from cortical bone, a shortcoming that limits its ability to detect changes within these skeletal compartments. An advantage of HRpQCT is that such separation is readily performed, and as indicated by our findings, HRpQCT imaging clearly demonstrates that MGUS is associated with significantly higher cortical porosity. Despite their larger radial bone size,3 MGUS patients had significantly lower cortical vBMD and tended to have thinner cortices relative to controls. These results extend our previous findings3 demonstrating altered trabecular microarchitecture with MGUS.
Another novel aspect of our analysis is the µFE models constructed to assess bone biomechanical properties in response to a simulated axial compression test.4 Our findings that failure load and stiffness both tended to be lower in MGUS patients are consistent with the suggestion that bone strength is reduced in MGUS. Notably, these deficits did not reach statistical significance, likely because of the compensatory increase in bone size seen in MGUS patients,3 an increase likely resulting from progressive periosteal (outer surface) bone apposition with concomitantly increased endocortical (inner surface) resorption, ultimately resulting in cortical thinning. Such outward cortical displacement increases resistance to bending stresses, providing a partial biomechanical adaptation to limit the overall loss of bone strength resulting from decreased cortical thickness.16 Consistent with this premise, MGUS patients had significantly lower radial apparent modulus (bone strength corrected for cross-sectional area) compared with controls.
The importance of cortical bone morphology in bone strength and fracture prevention is highlighted by observations that cortical bone comprises >80% of the adult skeleton,17 and that after age 65, most appendicular bone loss is cortical.18 Furthermore, 80% of fractures after age 65 predominantly occur at cortical skeletal sites.19 Thus, the cortical bone deterioration we observed in MGUS patients is of significant clinical concern and emphasizes the need for treatments that prevent such bone loss.
A limitation of our study is that currently only cross-sectional data are available. Thus, long-term consequences of the cortical bone abnormalities we observed in the MGUS patients remain unknown, although we plan to examine this question by longitudinally following this cohort. Another potential concern is that our HRpQCT measurements were limited to the radius. Finally, future studies are necessary to determine whether the identified skeletal abnormalities are worse in patients with multiple myeloma or related plasma cell malignancies.
In conclusion, despite their larger radial bone size, MGUS patients have compromised cortical microarchitecture (ie, increased cortical bone porosity) and reduced bone strength relative to controls. Our findings underscore the need to further delineate the factors that regulate bone microarchitecture in MGUS, both to better identify those patients at greatest fracture risk and to develop therapies to limit MGUS-associated bone loss.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank James M. Peterson for data management and Margaret Holets for performing the HRpQCT scans.
This work was supported by grants from the Mayo Hematologic Malignancies Program, a Mayo Career Development Award (M.T.D.), (K08 AR059138) (M.T.D), (T32 DK007352) (J.N.F), (AR027065), and the Center for Translational Science Activities (UL1TR000135).
Authorship
Contribution: J.N.F., W.Z., S.K.K., S.V.R., and M.T.D. designed the study; J.N.F., W.Z., A.C.N., L.K.M., and M.T.D. performed the research; R.M.J. contributed vital new analytic tools; J.N.F. and M.T.D. performed all statistical analyses; J.N.F., S.V.R., and M.T.D. wrote the manuscript; J.N.F., S.V.R., and M.T.D. had full access to study data and take responsibility for accuracy of data analysis; all authors were involved in the interpretation of data; and all authors read, provided comments, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew T. Drake, Mayo Clinic College of Medicine, 200 First St SW, Rochester, MN 55905; e-mail: drake.matthew@mayo.edu.