Key Points
Elderly females have a better outcome than elderly males and a more favorable rituximab pharmacokinetics than all other patients with DLBCL.
Prospective trials aiming at optimizing rituximab dose and schedule are warranted in all DLBCL patients.
Abstract
To determine the effect of gender on outcome, the male hazard ratio for progression-free survival (HRPFS-male) was determined in patients with diffuse large B-cell lymphoma (DLBCL). In young patients (MapThera International Trial study), HRPFS-male was 1.3 (P = .092) without and 1.1 (P = .660) with rituximab. In elderly patients (RICOVER-60 study), HRPFS-male was 1.1 (P = .348) with CHOP but increased to 1.6 (P = .004) with R-CHOP. The similar improvements of outcome in young patients were associated with similar rituximab clearances in young males and females (9.89 vs 10.38 mL/h; P = .238), whereas the greater benefit for elderly females was associated with a slower rituximab clearance (8.47 vs 10.59 mL/h; P = .005) and hence higher serum levels and longer exposure times, attributable to an age-dependent (P = .004) decrease of rituximab clearance in females but not males. Compared with elderly females, all other subgroups had significantly faster rituximab clearances and hence appear to be suboptimally dosed when rituximab is given at 375 mg/m2. Although early results of pharmacokinetic-based prospective trials designed to exploit the full therapeutic potential of rituximab suggest that increased doses and/or prolonged exposure times can improve the outcome of elderly males with DLBCL, further studies are warranted that address the optimization of rituximab dose and schedule in all subgroups of DLBCL patients.
Introduction
The outcome of patients with diffuse large B-cell lymphoma (DLBCL) significantly improved when standard cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) 211-3 or dose-dense CHOP-144 were combined with the monoclonal anti-CD20 antibody rituximab, and the combination of chemotherapy and rituximab is considered the standard treatment of DLBCL. Despite its widespread use in DLBCL, the manner in which rituximab is combined with CHOP in 2-week or 3-week schedules was established for practical and/or historical reasons and not based on pharmacokinetic data conveying the risk of suboptimal dosing. In one of the few studies on rituximab pharmacokinetics in DLBCL, we recently demonstrated that rituximab clearance was significantly reduced, rituximab serum levels increased, and exposure times were prolonged in elderly women compared with elderly men5 ; these gender-specific differences in rituximab pharmacokinetics in elderly DLBCL patients can explain why elderly male patients have a diminished benefit from the addition of rituximab and why male gender evolves as a significant risk factor in elderly patients treated with, but not without, rituximab. We have now extended our studies to young DLBCL patients and observed no increased risk of young males compared with young females with DLBCL when treated with rituximab. Interestingly, this was associated with similar rituximab pharmacokinetics in young female and male DLBCL patients. Our results indicate that subpopulations of DLBCL patients with faster rituximab clearance (and hence lower serum levels and shortened exposure times) have a reduced therapeutic benefit from the addition of rituximab, suggesting that the current standard dosing and/or scheduling of rituximab are suboptimal in these patients.
Patients, materials, and methods
Clinical data
Clinical data were used from the following prospective German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) studies in elderly patients with DLBCL: the RICOVER-60 (#NCT00052936) trial,4 the Pegfilgrastim trial (#NCT00726700),6 the RICOVER-60-noRTh study (#NCT 0052936),7 and the NHL-B2 trial.8 For young patients, clinical data were obtained from the MabThera International Trial (MInT) study (#NCT00400907)3 and from the MegaCHOEP (#NCT00129090) trial.9 In all these trials (supplemental Table 1; see the Blood Web site), patients received CHOP and CHOP-like CHOEP (CHOP plus etoposide) chemotherapy. All studies were performed in accordance with the Declaration of Helsinki. All protocols had been approved by the ethics committee of each participating institution, and all patients had given written informed consent.
Details of the statistical analysis have been described in the original reports of the respective trials.3,4,6-9 Event-free survival (EFS) was defined as the time from randomization to either disease progression, initiation of salvage therapy, additional (unplanned) treatments, relapse, or death from any cause; progression-free survival (PFS), as time from randomization to progression, relapse, or death from any cause; and overall survival (OS), as time from randomization to death from any cause. All tests for significance were 2-sided. Statistical analyses were performed with SPSS (version 11.5) and SAS 9.1.3 (SAS Institute Inc., Cary, NC).
Rituximab pharmacokinetics
The pharmacokinetic study of 20 elderly patients treated in the prospective multicenter RICOVER-604 and the rituximab-CHOP (R-CHOP)–14 Pegfilgrastim6 trials of the DSHNHL has been described in detail previously.5 For the present study, pharmacokinetic analyses were extended to 29 additional elderly patients who were treated in the DENSE-R-UP study (registered under EudraCT No. 2005-005229-68 and #NCT00290667) with CHOP-14 in combination with 8 administrations of rituximab every 14 days (female patients, 375 mg intravenously; male patients, 500 mg/m2). In addition, 33 younger patients were analyzed who received 6 administrations of rituximab every 2 (n = 18) or 3 (n = 15) weeks in combination with 6 cycles of CHOP-14 or CHOP-21, respectively, in the UNFOLDER study (Eudra CT No. 2005-005218-19; #NCT00278408). All patients included in the pharmacokinetic study had normal kidney and liver functions. Blood sampling and rituximab enzyme-linked immunosorbent assay were performed as described previously.5 Pharmacokinetic properties (population model building, pharmacokinetic analysis, and model validation) of rituximab were processed and analyzed using the nonlinear mixed-effect approach NONMEM as described previously.5 The statistical analysis of demographic data of the pharmacokinetic study was done using IBM SPSS version 19.0.0 2010 (SPSS Inc., Ehningen, Germany).
Results
Impact of gender on outcome of young and elderly DLBCL patients
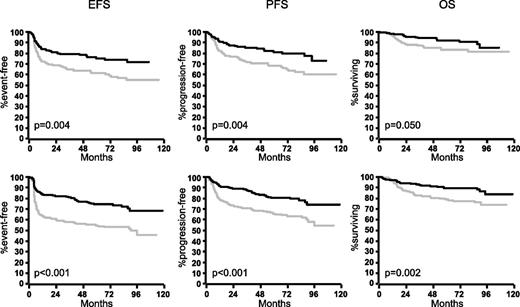
In the MInT study, young male patients benefited as much as young female patients from the addition of rituximab (Figure 1): rituximab improved 3-year EFS of males by 23% (from 58% to 81%), 3-year PFS by 18% (from 70% to 88%), and 3-year OS by 10% (from 83% to 93%). The respective figures for young females were 15% (from 64% to 79%) for EFS, 14% (from 71% to 85%) for PFS, and 7% (87% to 94%) for OS. In a multivariable analysis of young (good-prognosis) patients treated in the MInT study (Table 1), male patients had a slightly increased hazard if treated with CHOP (and CHOP-like) chemotherapy only, and this hazard did not increase for males treated with CHOP and CHOP-like regimens plus rituximab.
Outcome of female and male patients with DLBCL in the MInT study. Young good-prognosis (aaIPI = 0.1) male patients benefited at least as much as young good-prognosis females from the addition of rituximab. Upper row: females without rituximab (n = 189; gray curves) and females with rituximab (n = 156; black curves). Lower row: males without rituximab (n = 221; gray curves) and males with rituximab (n = 257; black curves). For multivariable analyses, see Table 1.
Outcome of female and male patients with DLBCL in the MInT study. Young good-prognosis (aaIPI = 0.1) male patients benefited at least as much as young good-prognosis females from the addition of rituximab. Upper row: females without rituximab (n = 189; gray curves) and females with rituximab (n = 156; black curves). Lower row: males without rituximab (n = 221; gray curves) and males with rituximab (n = 257; black curves). For multivariable analyses, see Table 1.
Multivariable analysis of risk factors for outcome in the MInT study (823 young good-prognosis [aaIPI = 0, 1] patients)
| Risk factor . | EFS . | PFS . | OS . | |||
|---|---|---|---|---|---|---|
| HR [95% CI] without rituximab (n = 410) . | HR [95% CI] with rituximab (n = 413) . | HR [95% CI] without rituximab (n = 410) . | HR [95% CI] with rituximab (n = 413) . | HR [95% CI] without rituximab (n = 410) . | HR [95% CI] with rituximab (n = 413) . | |
| Elevated LDH | 1.9 [1.3-2.8] (P < .001) | 1.3 [0.8-2.1] (P = .342) | 1.8 [1.2-2.7] (P = .005) | 1.7 [1.0-3.1] (P = .073) | 2.1 [1.2-3.5] (P = .006) | 1.8 [0.8-3.7] (P = .151) |
| Stages III and IV | 1.7 [1.1-2.4] (P = .008) | 1.9 [1.2-3.1] (P = .007) | 1.6 [1.1-2.5] (P = .027) | 2.3 [1.3-4.1] (P = .004) | 0.9 [0.5-1.8] (P = .852) | 1.8 [0.8-3.9] (P = .162) |
| >1 Extralymphatic site | 1.5 [0.9-2.5] (P = .102) | 0.8 [0.4-1.7] (P = .547) | 1.3 [0.7-2.3] (P = .346) | 0.6 [0.2-1.6] (P = .302) | 2.5 [1.2-5.2] (P = .014) | 0.5 [0.1-2.2] (P = .391) |
| Male vs female | 1.3 [1.0-1.8] (P = .092) | 1.1 [0.7-1.7] (P = .660) | 1.1 [0.8-1.5] (P = .632) | 1.1 [0.7-1.7] (P = .799) | 1.3 [0.8-2.1] (P = .244) | 1.2 [0.6-2.4] (P = .535) |
| Risk factor . | EFS . | PFS . | OS . | |||
|---|---|---|---|---|---|---|
| HR [95% CI] without rituximab (n = 410) . | HR [95% CI] with rituximab (n = 413) . | HR [95% CI] without rituximab (n = 410) . | HR [95% CI] with rituximab (n = 413) . | HR [95% CI] without rituximab (n = 410) . | HR [95% CI] with rituximab (n = 413) . | |
| Elevated LDH | 1.9 [1.3-2.8] (P < .001) | 1.3 [0.8-2.1] (P = .342) | 1.8 [1.2-2.7] (P = .005) | 1.7 [1.0-3.1] (P = .073) | 2.1 [1.2-3.5] (P = .006) | 1.8 [0.8-3.7] (P = .151) |
| Stages III and IV | 1.7 [1.1-2.4] (P = .008) | 1.9 [1.2-3.1] (P = .007) | 1.6 [1.1-2.5] (P = .027) | 2.3 [1.3-4.1] (P = .004) | 0.9 [0.5-1.8] (P = .852) | 1.8 [0.8-3.9] (P = .162) |
| >1 Extralymphatic site | 1.5 [0.9-2.5] (P = .102) | 0.8 [0.4-1.7] (P = .547) | 1.3 [0.7-2.3] (P = .346) | 0.6 [0.2-1.6] (P = .302) | 2.5 [1.2-5.2] (P = .014) | 0.5 [0.1-2.2] (P = .391) |
| Male vs female | 1.3 [1.0-1.8] (P = .092) | 1.1 [0.7-1.7] (P = .660) | 1.1 [0.8-1.5] (P = .632) | 1.1 [0.7-1.7] (P = .799) | 1.3 [0.8-2.1] (P = .244) | 1.2 [0.6-2.4] (P = .535) |
Not adjusted for Eastern Cooperative Oncology Group Performance Status (ECOG) performance state because only 6 of the 823 patients had an ECOG >1.
CI, confidence interval; LDH, lactate dehydrogenase.
A similar pattern was observed in 262 young poor-prognosis (age adjusted International Prognostic Index [aaIPI] = 2, 3) patients treated in the MegaCHOEP trial9 (Table 2): the male hazard was 1.3 (0.8-1.9; P = .258), 1.2 (0.7-1.8; P = .521), and 1.0 (0.6-1.6; P = .898) for EFS, PFS, and OS, respectively, thus being similar to the hazard ratios (HRs) observed in the young good-prognosis population of the MInT study with and without rituximab.
Multivariable analysis of risk factors for outcome in the MegaCHOEP study (262 young poor-prognosis [aaIPI = 2, 3] patients)
| Risk factor . | EFS . | PFS . | OS . | |||
|---|---|---|---|---|---|---|
| HR [95% CI] without rituximab (n = 0) . | HR [95% CI] with rituximab (n = 262) . | HR [95% CI] without rituximab (n = 0) . | HR [95% CI] with rituximab (n = 262) . | HR [95% CI] without rituximab (n = 0) . | HR [95% CI] with rituximab (n = 262) . | |
| Elevated LDH | - | 1.1 [0.4-3.8] (P = .818) | - | 1.0 [0.3-3.2] (P = .957) | - | 1.2 [0.3-5.3] (P = .778) |
| ECOG performance state >1 | - | 1.6 [1.0-2.5] (P = .034) | - | 1.8 [1.1-2.9] (P = .019) | - | 1.8 [1.0-3.2] (P = .040) |
| Stages III and IV | - | 2.8 [0.7-11.7] (P = .162) | - | 2.4 [0.6-10.0] (P = .245) | - | 3.3 [0.4-24.6] (P = .250) |
| >1 Extralymphatic site | - | 1.2 [0.8-1.8] (P = .357) | - | 1.2 [0.8-1.9] (P = .413) | - | 1.0 [0.6-1.8] (P = .953) |
| Male vs female | - | 1.3 [0.8-2.0] (P = .258) | - | 1.2 [0.7-1.9] (P = .521) | - | 1.0 [0.6-1.7] (P = .900) |
| Risk factor . | EFS . | PFS . | OS . | |||
|---|---|---|---|---|---|---|
| HR [95% CI] without rituximab (n = 0) . | HR [95% CI] with rituximab (n = 262) . | HR [95% CI] without rituximab (n = 0) . | HR [95% CI] with rituximab (n = 262) . | HR [95% CI] without rituximab (n = 0) . | HR [95% CI] with rituximab (n = 262) . | |
| Elevated LDH | - | 1.1 [0.4-3.8] (P = .818) | - | 1.0 [0.3-3.2] (P = .957) | - | 1.2 [0.3-5.3] (P = .778) |
| ECOG performance state >1 | - | 1.6 [1.0-2.5] (P = .034) | - | 1.8 [1.1-2.9] (P = .019) | - | 1.8 [1.0-3.2] (P = .040) |
| Stages III and IV | - | 2.8 [0.7-11.7] (P = .162) | - | 2.4 [0.6-10.0] (P = .245) | - | 3.3 [0.4-24.6] (P = .250) |
| >1 Extralymphatic site | - | 1.2 [0.8-1.8] (P = .357) | - | 1.2 [0.8-1.9] (P = .413) | - | 1.0 [0.6-1.8] (P = .953) |
| Male vs female | - | 1.3 [0.8-2.0] (P = .258) | - | 1.2 [0.7-1.9] (P = .521) | - | 1.0 [0.6-1.7] (P = .900) |
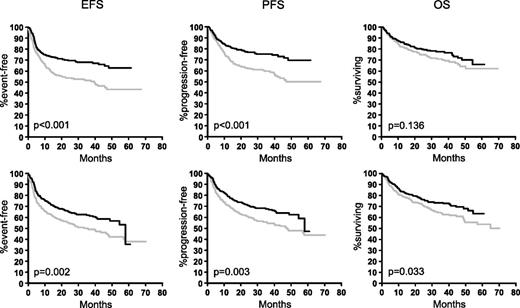
In contrast, the benefit obtained by the addition of rituximab in 1222 elderly patients treated within the RICOVER-60 trial with CHOP-14 ± rituximab was different in female and male patients. As can be seen from Figure 2, both elderly females and males benefited from the addition of rituximab, but the improvement was greater in elderly females. When treated with CHOP-14 only, female patients had a slightly better outcome than males, despite the fact that they presented with a worse prognostic profile. However, after the addition of rituximab, the differences between female and male patients were more pronounced. Rituximab improved the 3-year EFS in elderly males by 13% (from 49% to 62%), 3-year PFS by 13% (from 55% to 68%), and 3-year OS by 10% (from 63% to 73%). The respective figures for elderly females were 17% (from 51% to 68%) for EFS, 15% (from 60% to 75%) for PFS, and 7% (from 71% to 78%) for OS. When adjusted for the IPI risk factors and age >70 in a multivariable Cox regression analysis (Table 3), the HRmale without rituximab for EFS, PFS, and OS was 1.1, 1.1, and 1.3, respectively, and significant only for OS (P = .038); whereas it was significant after the addition of rituximab for EFS (1.5; P = .004), PFS (1.7; P = .001), and OS (154; P = .01).
Outcome of female and male patients in the RICOVER-60 study. Elderly female patients (upper graphs) benefited more from the addition of rituximab than elderly males (lower graphs). Upper row: female patients without rituximab (n = 287; gray curves) and female patients with rituximab (n = 285; black curves). Lower row: males without rituximab (n = 325; gray curves) and males with rituximab (n = 325; black curves).
Outcome of female and male patients in the RICOVER-60 study. Elderly female patients (upper graphs) benefited more from the addition of rituximab than elderly males (lower graphs). Upper row: female patients without rituximab (n = 287; gray curves) and female patients with rituximab (n = 285; black curves). Lower row: males without rituximab (n = 325; gray curves) and males with rituximab (n = 325; black curves).
Multivariable analysis of risk factors for outcome in the RICOVER-60 study (1222 elderly patients)
| Risk factor . | EFS . | PFS . | OS . | |||
|---|---|---|---|---|---|---|
| HR [95% CI] without rituximab (n = 612) . | HR [95% CI] with rituximab (n = 610) . | HR [95% CI] without rituximab (n = 612) . | HR [95% CI] with rituximab (n = 610) . | HR [95% CI] without rituximab (n = 612) . | HR [95% CI] with rituximab (n = 610) . | |
| Elevated LDH | 1.5 [1.1-1.9] (P = .004) | 1.7 [1.2-2.3] (P = .001) | 1.6 [1.2-2.2] (P = .001) | 2.1 [1.4-2.9] (P < .001) | 1.8 [1.3-2.4] (P = .001) | 1.8 [1.3-2.7] (P < .001) |
| ECOG performance state >1 | 1.8 [1.4-2.5] (P = .001) | 1.6 [1.1-2.3] (P = .001) | 1.7 [1.2-2.3] (P = .001) | 1.6 [1.4-2.9] (P = .016) | 1.8 [1.3-2.5] (P < .001) | 1.9 [1.1-2.5] (P = .009) |
| Stages III and IV | 1.6 [1.2-2.1] (P = .001) | 1.5 [1.1-2.1] (P = .008) | 2.0 [1.5-2.7] (P < .001) | 1.5 [1.0-2.1] (P = .031) | 1.6 [1.2-2.3] (P = .005) | 1.6 [1.1-2.3] (P = .024) |
| >1 Extralymphatic site | 1.4 [1.1-1.9] (P = .017) | 1.0 [0.7-1.4] (P = .958) | 1.7 [1.2-2.2] (P = .001) | 1.1 [0.7-1.6] (P = .672) | 1.7 [1.2-2.4] (P = .002) | 1.1 [0.7-1.6] (P = .817) |
| Age >70 y | 1.2 [1.0-1.6] (P = .1) | 1.6 [1.2-2.1] (P = .002) | 1.4 [1.5-1.7] (P = .02) | 1.6 [1.2-2.2] (P = .003) | 1.6 [1.2-2.2] (P = .001) | 2.0 [1.5-2.9] (P < .001) |
| Male vs female | 1.1 [0.8-1.3] (P = .644) | 1.5 [1.2-2.0] (P = .004) | 1.1 [0.9-1.5] (P = .313) | 1.7 [1.2-2.3] (P = .001) | 1.4 [1.0-1.8] (P = .038) | 1.5 [1.1-2.2] (P = .01) |
| Risk factor . | EFS . | PFS . | OS . | |||
|---|---|---|---|---|---|---|
| HR [95% CI] without rituximab (n = 612) . | HR [95% CI] with rituximab (n = 610) . | HR [95% CI] without rituximab (n = 612) . | HR [95% CI] with rituximab (n = 610) . | HR [95% CI] without rituximab (n = 612) . | HR [95% CI] with rituximab (n = 610) . | |
| Elevated LDH | 1.5 [1.1-1.9] (P = .004) | 1.7 [1.2-2.3] (P = .001) | 1.6 [1.2-2.2] (P = .001) | 2.1 [1.4-2.9] (P < .001) | 1.8 [1.3-2.4] (P = .001) | 1.8 [1.3-2.7] (P < .001) |
| ECOG performance state >1 | 1.8 [1.4-2.5] (P = .001) | 1.6 [1.1-2.3] (P = .001) | 1.7 [1.2-2.3] (P = .001) | 1.6 [1.4-2.9] (P = .016) | 1.8 [1.3-2.5] (P < .001) | 1.9 [1.1-2.5] (P = .009) |
| Stages III and IV | 1.6 [1.2-2.1] (P = .001) | 1.5 [1.1-2.1] (P = .008) | 2.0 [1.5-2.7] (P < .001) | 1.5 [1.0-2.1] (P = .031) | 1.6 [1.2-2.3] (P = .005) | 1.6 [1.1-2.3] (P = .024) |
| >1 Extralymphatic site | 1.4 [1.1-1.9] (P = .017) | 1.0 [0.7-1.4] (P = .958) | 1.7 [1.2-2.2] (P = .001) | 1.1 [0.7-1.6] (P = .672) | 1.7 [1.2-2.4] (P = .002) | 1.1 [0.7-1.6] (P = .817) |
| Age >70 y | 1.2 [1.0-1.6] (P = .1) | 1.6 [1.2-2.1] (P = .002) | 1.4 [1.5-1.7] (P = .02) | 1.6 [1.2-2.2] (P = .003) | 1.6 [1.2-2.2] (P = .001) | 2.0 [1.5-2.9] (P < .001) |
| Male vs female | 1.1 [0.8-1.3] (P = .644) | 1.5 [1.2-2.0] (P = .004) | 1.1 [0.9-1.5] (P = .313) | 1.7 [1.2-2.3] (P = .001) | 1.4 [1.0-1.8] (P = .038) | 1.5 [1.1-2.2] (P = .01) |
The higher hazard for elderly male patients after R-CHOP compared with CHOP was not only observed in the RICOVER-60 trial, but was also confirmed in other trials with elderly DLBCL patients: without rituximab, the male hazard for an event in PFS ranged from 1.1 and 1.2 in trials or treatment arms without rituximab, whereas it ranged from 1.5 to 3.5 in trials or treatment arms with rituximab (Table 410 ). The overall male hazard for all 831 elderly patients treated without rituximab in these trials was 1.2 (0.9-1.5) and not significant (P = .203), whereas it was 1.6 (1.2-2.1) for 830 patients treated with rituximab, a highly significant (P < .001) hazard confirming the findings obtained from the RICOVER-60 trial alone. In summary, elderly male patients benefit significantly less from the addition of rituximab than females.
HRs of elderly male vs female patients for an event in PFS in trials and treatment arms with and without rituximab, respectively
| Trial . | HR [95% CI]* without rituximab . | HR [95% CI]* with rituximab . |
|---|---|---|
| RICOVER-604 (n = 1222) | 1.1 [0.9-1.4] (n = 612; P = .348) | 1.6 [1.2-2.2] (n = 610; P = .004) |
| Pegfilgrastim trial6 (n = 103) | 1.2 [0.4-3.3] (n = 47; P = .783) | 3.5 [1.0-12.8] (n = 56; P = .055) |
| NHL-B2 (CHOP-14)8 (n = 172) | 1.2 [0.8-1.9] (n = 172; P = .420) | - |
| RICOVER-noRTh10 (n = 164) | - | 1.5 [0.9-2.5] (n = 164; P = .196) |
| All studies CHOP-14 (n = 1661) | 1.2 [0.9-1.5] (n = 831; P = .203) | 1.6 [1.2-2.1] (n = 830; P < .001) |
| Trial . | HR [95% CI]* without rituximab . | HR [95% CI]* with rituximab . |
|---|---|---|
| RICOVER-604 (n = 1222) | 1.1 [0.9-1.4] (n = 612; P = .348) | 1.6 [1.2-2.2] (n = 610; P = .004) |
| Pegfilgrastim trial6 (n = 103) | 1.2 [0.4-3.3] (n = 47; P = .783) | 3.5 [1.0-12.8] (n = 56; P = .055) |
| NHL-B2 (CHOP-14)8 (n = 172) | 1.2 [0.8-1.9] (n = 172; P = .420) | - |
| RICOVER-noRTh10 (n = 164) | - | 1.5 [0.9-2.5] (n = 164; P = .196) |
| All studies CHOP-14 (n = 1661) | 1.2 [0.9-1.5] (n = 831; P = .203) | 1.6 [1.2-2.1] (n = 830; P < .001) |
Relative risk in multivariable analyses adjusting for the IPI risk factors (elevated LDH, stage III/IV, and >1 extralymphatic site of involvement).
Correlation with rituximab pharmacokinetics
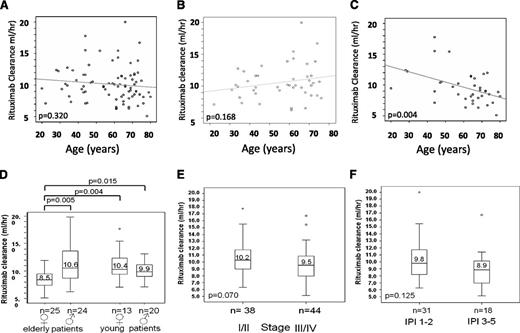
The differences in outcome of elderly female and male patients with and without rituximab cannot be explained by differences with respect to relative doses or dose intensities of myelosuppressive drugs because these were identical in the arms with and without rituximab and identical for females and males: the relative doses for cyclophosphamide were 98% for both genders, whereas for doxorubicin, the doses were 98% for females and 96% for males (supplemental Figure 1). Elderly females had more grade 4 leukocytopenia (without rituximab, 39.0% vs 25.2%, P < .001; with rituximab, 43.7% vs 22.5%, P < .001), but fewer infections of grade 3 and 4 (without rituximab, 9% vs 14.5%, P = .036; with rituximab, 9.4% vs 8.4%, P = .056); whereas there was no difference between elderly females and males with respect to fever (without rituximab, 13.4% vs 16.5%, P = .325; with rituximab, 10.5% vs 13.5%, P = .185). Similarly, there were no differences between elderly females and males with respect to the received doses of rituximab (supplemental Figure 2). As shown in Figure 3A, there was no correlation between age and rituximab clearance when all 82 patients were analyzed (–0.1; P = .320). When analyzed according to age and gender, there was a nonsignificant positive correlation in 44 male patients with increasing age (+0.2; P = .168; Figure 3B), but a significant negative correlation between age and rituximab clearance in female patients (–0.5; P = .004; Figure 3C). This resulted in a median rituximab clearance of 8.47 mL/hour in elderly females, which was significantly lower than in elderly males (10.59; P = .005), as well as compared with young females (10.38 mL/hour; P = .004) and young males (9.89 mL/hour; P = .015; Figure 3D).
Effect of age, gender, tumor load, and IPI on rituximab clearance. No correlation of rituximab clearance with age was observed when all patients were analyzed (A). However, when female and male patients were analyzed separately, clearance slightly (nonsignificantly) increased in males (B) and significantly decreased in females (C) with increasing age. As a result, differences in rituximab clearance were not found between young female and male patients but were significant between elderly female and elderly male patients, elderly and young female patients, and elderly female and young male patients (D). No significant differences in rituximab clearance were found between patients with low tumor burden (stages I/II) and high tumor burden (stages II/IIII; E), and no differences were observed between (elderly) good-prognosis and poor-prognosis patients (F); for the latter analysis, only elderly patients were evaluated because no pharmacokinetic data were available from young poor-prognosis patients.
Effect of age, gender, tumor load, and IPI on rituximab clearance. No correlation of rituximab clearance with age was observed when all patients were analyzed (A). However, when female and male patients were analyzed separately, clearance slightly (nonsignificantly) increased in males (B) and significantly decreased in females (C) with increasing age. As a result, differences in rituximab clearance were not found between young female and male patients but were significant between elderly female and elderly male patients, elderly and young female patients, and elderly female and young male patients (D). No significant differences in rituximab clearance were found between patients with low tumor burden (stages I/II) and high tumor burden (stages II/IIII; E), and no differences were observed between (elderly) good-prognosis and poor-prognosis patients (F); for the latter analysis, only elderly patients were evaluated because no pharmacokinetic data were available from young poor-prognosis patients.
Impact of tumor load and IPI on rituximab clearance
In contrast to age, there was no significant difference between low and high tumor burden with respect to rituximab clearance (Figure 3E). Contrary to what one might have expected and what has been reported for follicular lymphoma,11 median rituximab clearance in stage I/II patients was faster than in patients with stage III/IV (10.23 mL/hour vs 9.50 mL/hour; P = .070), but this can be explained by the enrichment of elderly females with advanced stage in this analysis. The same applies when low- and low/intermediate-risk patients according to the IPI were compared with intermediate/high- and high-risk patients (Figure 3F). The latter analysis was restricted to elderly patients because all the young patients from whom pharmacokinetic data were available belonged to the good-prognosis group (aaIPI = 0, 1).
Discussion
The impact of gender on outcome of patients with DLBCL has been discussed controversially.12-15 From nonrandomized comparisons, it appears that the negative impact of male gender is more pronounced in DLBCL patients treated with R-CHOP–like chemotherapy than with chemotherapy alone.16,17 The multivariable analyses of RICOVER-604 show that male gender is associated with a slightly (but nonsignificantly) increased risk for an event in EFS, PFS, and OS in patients treated with CHOP chemotherapy without rituximab, but male gender evolves as a significant risk factor for EFS and PFS when rituximab is added to CHOP, demonstrating a differential effect of rituximab on the outcome of elderly female and male patients. This observation in the RICOVER-60 trial was confirmed in the other trials listed in Table 2, where elderly patients received CHOP with and without rituximab. That this did not translate into significant differences of OS is probably attributable to the fact that elderly males in the RICOVER-60 study who were resistant to or relapsed after R-CHOP only fared better than elderly females, most likely because elderly males received salvage treatment containing high-dose chemotherapy and stem cell transplantation more often than elderly females (N.S.; manuscript in preparation).
That male gender is a significant risk factor in elderly patients treated with rituximab has recently been confirmed in an Italian-Brazilian study.18 In RICOVER-60, this cannot be explained by differences with respect to the received doses of chemotherapy or rituximab (supplemental Figures 1 and 2), which were close to 100% of the planned doses, leaving the differences in rituximab clearance (and hence rituximab serum levels and exposure times) the only association with and explanation for the different outcome of elderly females and males.
The most important finding of this study is the fact that the commonly accepted increased male risk in DLBCL treated with rituximab was confirmed for elderly, but not for young patients, who benefited at least as much as young females from the addition of rituximab to CHOP. Because the cytotoxic effect of rituximab on the malignant cells was as strong in young males as in young females, there are obviously no gender-associated differences in sensitivity of female and male lymphoma cells to the cytotoxic effects mediated by rituximab; rather, the different effects of gender on outcome of young and elderly patients are attributable to differences in pharmacokinetics: significant differences of rituximab pharmacokinetics were not observed in young DLBCL patients, where males and females benefited as much from the addition of rituximab and where the addition of rituximab did not increase the male outcome hazard. However, because rituximab clearances between males and females drift apart with increasing age, elderly females have a highly significantly slower rituximab clearance (and hence higher serum levels and longer exposure times) compared with elderly males. Quite obviously, elderly males with their comparatively fast rituximab clearance are suboptimally dosed when rituximab is given at 375 mg/m2 body surface area, and the lower rituximab serum levels and/or the shorter exposure times are the most likely reason for the inferior outcome of elderly males compared with females once rituximab is added.
The age-dependent decrease of the rituximab clearance in women is mostly likely attributable to significant changes in rituximab metabolism, which is supposed to be degraded in the liver and other organs by a process of nonspecific catabolism.19-21 We found only 1 similar observation in the literature: in an investigation of age- and gender-dependent effects on pharmacokinetics of lanoteplase, a plasminogen activator, a gender-dependent effect on total clearance was observed with elderly females having a 32% lower mean clearance than elderly males.22
Rituximab clearance paralleled the outcome of young and elderly DLBCL patients: in young patients, rituximab clearances (and hence rituximab serum half-lives, serum levels and exposure times) are not different, and no differential outcome was observed with respect to gender after CHOP(-like) chemotherapy plus rituximab compared with CHOP(-like) chemotherapy without rituximab in the MInT23 study for young good-prognosis patients and the MegaCHOEP9 study for young poor-prognosis patients.
The findings of our study are not only helpful for the interpretation of recent DLBCL trials, but should also impact on the design of rituximab-containing regimens in the future. If serum levels are important, as has been suggested from a study in relapsed DLBCL,24 increasing the dose of rituximab would be the appropriate strategy, and early follow-up of the DENSE-R-CHOP-14 trial25 suggests that this strategy might work in elderly high-risk patients. Some of the most convincing data that pharmacokinetic-based adaptations of the rituximab administration can indeed improve the outcome of patients with an unfavorable rituximab pharmacokinetics come from the results of the SMARTE-R-CHOP-14 trial of the DSHNHL,26 where elderly patients received 8 doses of rituximab together with 6 doses of CHOP-14 over a period of 240 days. Interestingly, the 3-year OS improvement by 13% in the SMARTE-R-CHOP-14 high-risk population compared with the same population in the RICOVER-60 study was attributable to a 20% improvement in poor-prognosis (IPI = 3-5) male patients, with an improvement of only 4% in elderly poor-prognosis female patients. Obviously, elderly male patients with their faster rituximab clearance benefited particularly from the prolonged rituximab exposure time in the SMARTE-R-CHOP-14 trial. Most importantly, the extended rituximab exposure time in the SMARTE-R-CHOP-14 trial completely eliminated the outcome differences between elderly female and male patients, strongly suggesting for the first time that the increased hazard of elderly males with DLBCL can indeed be neutralized by pharmacokinetic consequences derived from the observations made in this study (ie, longer exposure time in the SMARTE-R-CHOP-14 trial).26
Quite obviously, elderly males appear to be suboptimally dosed with 375 mg/m2 rituximab given synchronously with CHOP because we have the comparison with the better results in elderly females; however, we do not even know if this rituximab dose (and schedule) is optimal for elderly female patients with DLBCL or if it can be improved further by higher rituximab doses or longer rituximab exposure times. Because all other subgroups of patients have a significantly faster clearance than elderly females, one must assume that not only elderly males, but also young females and young males are suboptimally dosed compared with elderly females. Therefore, further trials are warranted to optimize the dose and schedule of rituximab in DLBCL in all subpopulations of DLBCL. Such trials should be feasible because of the large therapeutic window of rituximab where no significant increase in toxicity has been observed with single doses up to 2250 mg/m2 (in patients with chronic lymphocytic leukemia).27 As a corollary, novel monoclonal CD20, which is higher dosed than rituximab in ongoing clinical studies, should be compared with rituximab at the same dose and schedule in order to yield meaningful results concerning the relative efficacy of the respective antibodies.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the DSHNHL Central Office at the Department of Internal Medicine I, Saarland University Medical School in Homburg for their support. We thank Beate Mann and Katja Rillich (Institute for Medical Informatics, Statistics, and Epidemiology Leipzig) for expert technical assistance.
This work was supported by Deutsche Krebshilfe (a charity organization). The cost for the pharmacokinetic studies was covered by Roche.
Authorship
Contribution: M.P., N.M., and C.M. designed the study; S.Z. and E.K. performed the statistical analysis; C.M. and M.H.J.W. performed the pharmacokinetic studies; M.R. and N.S. provided clinical data; G.H., T.R., and V.P. assembled and analyzed pharmacokinetic and clinical data; and all authors participated in writing the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.P. is on the advisory boards of Boehringer Ingelheim, Celgene, Pfizer, Onyx, and Roche and has received research support from Amgen and Roche. The remaining authors declare no competing financial interests.
Correspondence: Michael Pfreundschuh, Klinik für Innere Medizin I, Saarland University Medical School, D-66424 Homburg (Saar), Germany; e-mail: michael.pfreundschuh@uks.eu.