In this issue of Blood, Thompson et al reveal a key role for hypoxia-inducible factor (HIF)-2α in the adaptation of neutrophils to hypoxia. Tissue hypoxia is a common feature of trauma and inflammation. Infiltrating neutrophils must adapt to this low-oxygen environment to satisfy the metabolic and functional demands of an immune response.1
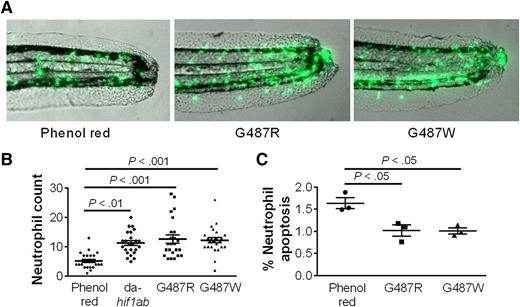
HIF-2α increases neutrophil life span. (A-B) Human-equivalent HIF-2α gain-of-function mutations in zebra fish impair the clearance of green fluorescent protein–labeled neutrophils after a tail fin transection. (C) The increased neutrophil number is associated with a decrease in TUNEL-positive apoptotic neutrophils. See the complete Figure 3 in the article by Thompson et al that begins on page 366.
HIF-2α increases neutrophil life span. (A-B) Human-equivalent HIF-2α gain-of-function mutations in zebra fish impair the clearance of green fluorescent protein–labeled neutrophils after a tail fin transection. (C) The increased neutrophil number is associated with a decrease in TUNEL-positive apoptotic neutrophils. See the complete Figure 3 in the article by Thompson et al that begins on page 366.
Much is known about the factors controlling neutrophil production and the recruitment of neutrophils to sites of inflammation. In contrast, the factors controlling the survival and clearance of neutrophils from tissues remain poorly defined. The net accumulation of neutrophils at sites of inflammation will be a function of recruitment and median neutrophil life span. Hypoxia increases neutrophil survival,2 suggesting that hypoxia may impair the resolution of neutrophilic inflammatory disorders such as acute respiratory distress syndrome, bronchiolitis obliterans organizing pneumonia, and respiratory syncytial virus infection. The oxygen-sensitive HIF/von Hippel-Lindau pathway is fundamental to hypoxia adaptation. The HIF-1α and HIF-2α transcription factors are both induced by hypoxia in neutrophils.3 However, HIF-1α and HIF-2α expression are not always coordinately regulated, and they can serve distinct functions in other cell types. Thompson and colleagues now demonstrate key roles for HIF-2α in extending neutrophil life span in zebra fish, mice, and humans.1
Humans with gain-of-function mutations in HIF-2α develop erythrocytosis because of exaggerated erythropoietin production4 but are not known to be predisposed to neutrophilic inflammatory disease. Mice with targeted HIF-2α point mutations recapitulate the human syndrome of erythrocytosis.5 Iron chelation with deferoxamine stabilizes HIF-1α and HIF-2α in neutrophils, but clinical use of this compound is also not associated with neutrophilic inflammatory disease (however, other iron chelators were not studied).1 Increased HIF-2α messenger RNA (mRNA) and protein were found in neutrophils from patients with chronic obstructive pulmonary disease (COPD) and inflammatory arthritis (IA). This chronic HIF-2α expression may elicit unique biological effects not seen in normoxic human neutrophils from patients with a HIF-2α gain-of-function mutation.
Inflammatory cytokines and Toll-like receptor ligands present in COPD and IA may be inducing HIF-2α expression independently of hypoxia. Consistently, patients with gain-of-function HIF-2α mutations express more HIF-2α target genes at steady state, suggesting that hypoxia is not always required for the induction or function of HIF-2α in neutrophils. HIF-2α can antagonize the expression of HIF-1α in nonhematopoietic cells, but it is not known if similar regulatory mechanisms exist in neutrophils.6 Previous studies in macrophages demonstrate that lipopolysaccharide can induce HIF-1α mRNA expression but that hypoxia is a prerequisite for HIF-1α protein accumulation via the inhibition of prolyl hydroxylases.7 Like HIF-1α, the accumulation of HIF-2α protein at sites of inflammation may therefore be dependent on factors controlling both gene transcription and HIF-2α protein half-life.
The key role of HIF-2α in the regulation of macrophage inflammatory cytokine production introduces a possible caveat to this study because the mice used in these studies lacked HIF-2α in neutrophils and macrophages. This was addressed using a fractionated irradiation regimen (3 fractions of 1 Gy/day for 4 days) to ablate bone marrow–derived myeloid cells but not wild-type resident lung macrophages. Following acute lung injury, HIF-2α–deficient neutrophils were recruited to lungs containing wild-type resident macrophages. This recruitment occurred independently of HIF-2α, but the subsequent resolution of neutrophilic inflammation was accelerated in the absence of HIF-2α. Conversely, expression of gain-of-function HIF-2α mutants in zebra fish delayed the clearance of neutrophils from a tail fin wound (see figure). Moreover, HIF-2α deficiency affects neutrophil life span but not phagocytosis or the respiratory burst, suggesting that the diverse functional roles of HIF-2α in macrophages do not extend to neutrophils.8 Inflammatory cytokine production by HIF-2α–deficient macrophages and neutrophils was also not altered in an acute lung injury model, supporting the conclusion that the alterations in neutrophil life span are cell intrinsic.
This study reports that human HIF-2α gain-of-function mutations increase basal expression of prolyl hydroxylase-3 (PHD3), an enzyme that helps neutrophils adapt to hypoxia. PHD3 is upregulated in peripheral blood neutrophils from patients with rheumatoid arthritis.9 This study demonstrates that HIF-2α is also strongly upregulated in peripheral blood neutrophils and lung biopsies from patients with rheumatoid arthritis and COPD, respectively, suggesting that HIF-2α regulates PHD3 expression in these human cells. PHD3 regulates Bcl-xL, a prosurvival protein whose expression is responsive to hypoxia.9 However, in this study, no perturbations were noted in Bcl-xL expression in HIF-2α–deficient neutrophils. More studies are needed to determine if Bcl-xL, or other prosurvival proteins such as Mcl-1, are HIF-2α targets during the adaptation to hypoxia. Neutrophil-specific conditional gene-targeting approaches will be essential to identify host factors that shift the balance between neutrophil life and death.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal