In this issue of Blood, Tezuka et al report the establishment of humanized mice infected by human T-cell leukemia virus type 1 (HTLV-1) that recapitulate adult T-cell leukemia (ATL)-like leukemic symptoms and display HTLV-1–specific adaptive immune responses.1
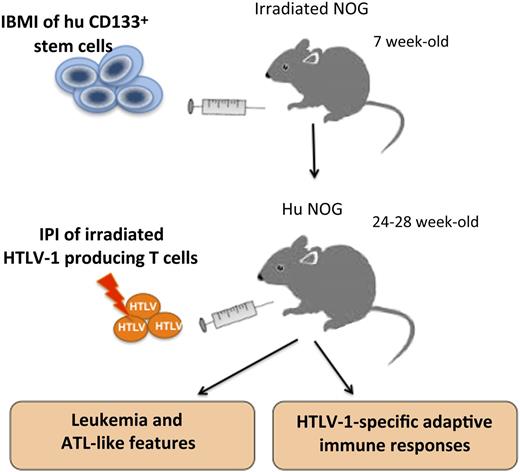
Schematic representation of the humanization and infection procedure according to Tezuka et al. Human CD133+ stem cells purified from HLA-A2-positive cord blood donors are injected into the bone marrow of 7-week-old NOG mice. By 24 to 28 weeks of age, irradiated HTLV-1–producing cells were intraperitoneally injected (IPI) into IBMI-huNOG mice. During the following 7 to 25 weeks, proliferation of CD25+CD4+ T cells and ATL-like features were observed in infected mice. Interestingly, these infected mice developed cytotoxic T lymphocytes as well as antibodies against HTLV-1 antigens.
Schematic representation of the humanization and infection procedure according to Tezuka et al. Human CD133+ stem cells purified from HLA-A2-positive cord blood donors are injected into the bone marrow of 7-week-old NOG mice. By 24 to 28 weeks of age, irradiated HTLV-1–producing cells were intraperitoneally injected (IPI) into IBMI-huNOG mice. During the following 7 to 25 weeks, proliferation of CD25+CD4+ T cells and ATL-like features were observed in infected mice. Interestingly, these infected mice developed cytotoxic T lymphocytes as well as antibodies against HTLV-1 antigens.
HTLV-1 is a deltaretrovirus that is etiologically linked to ATL, an aggressive malignancy characterized by the monoclonal expansion of leukemic CD25+CD4+ T cells (with atypical multilobulated nuclei) in the peripheral blood. ATL, which is mainly observed in a small percentage of individuals infected during the neonatal period, has a poor prognosis when it reaches an acute state.2
The observations reported by Tezuka et al present an interesting and original contribution of mouse models for biomedical research.3 During the last 5 decades, intensive efforts to generate mouse models that allow a long-term reconstitution of the human hematolymphoid system (hHLS) in vivo have been achieved by successful genetic modifications of severe combined immunodeficient (scid) mice and by improving the engraftment procedures for human stem cells (hSCs).4,5 These hHLS mice have therefore become indispensable models for evaluating the pathogenesis of human lymphotropic viruses such as HIV, Epstein-Barr virus (EBV), and HTLV-1.
Thus, a pioneering study has shown that reconstitution of T lymphopoiesis with HTLV-1–infected CD34+ cells injected into scid mice engrafted with human thymus and liver tissues resulted in the alteration of thymopoiesis.6 Later on, Banerjee et al7 reported that nonobese diabetic/SCID and NOD/LtSz-scid/IL-2Rγc−/− (NSG) mice infected through the inoculation of CD34+ hSCs, ex vivo infected by HTLV-1, partially recapitulate HTLV-1–induced T-cell leukemogenesis. More recently, newborn BALB/c/Rag2−/−IL-2Rγc−/− (BRG) mice intrahepatically inoculated with CD34+ hSCs have been infected by HTLV-1 6 to 8 weeks later through intraperitoneal inoculation of lethally irradiated HTLV-1–producing T cells.8 Under these conditions, T-cell development in the thymus of these mice is profoundly altered, which correlates with an increased level of the proviral load (PVL), the expansion of mature CD25+CD4+ T cells, and the development of ATL-like pathologic features, such as splenomegaly and lymphoma, phenotypically similar to those found in ATL patients.
The observations of Tezuka et al confirm and extend those reported by these previous studies. It is worth noting that Tezuka et al have chosen a different strategy of humanization by performing intra-bone marrow injection (IBMI) of cord blood CD133+ hSCs into 7-week-old NOG (NOD/Shi-scid/IL-2Rγc−/− null) mice. One month after injection, the majority of human CD45+ (hCD45+) lymphocytes present in the bone marrow of these humanized (huNOG) mice consisted of CD19+ B cells. Three to 5 months after engraftment, the number of human CD3+ T cells and the CD4+:CD8+ ratio increased and reached stable levels in the periphery. Likewise, a stable ratio of B cells to T cells in the peripheral blood of these mice indicated the formation of a stable immune system.
Accordingly, Tezuka et al elected to infect huNOG mice 6 to 7 months after engraftment by intraperitoneal injection of lethally irradiated HTLV-1–producing T cells (see figure). As soon as 4 to 6 weeks later, they observed an increased number of hCD45+ cells in the peripheral blood. Then, in about 38% of infected mice, a marked expansion of CD25+CD4+ T cells in the spleen was found to correlate with a high PVL, ATL-like leukemic features (such as splenomegaly), presence of abnormal leukemic T cells with lobulated nuclei in the periphery, and downregulation of CD3 expression. In addition, they observed an oligoclonal proliferation of human T cells in these infected animals.
Interestingly, the main observation of the study by Tezuka et al1 concerns the development of HTLV-1–specific adaptive immune responses. First, they observed the presence of restricted cytotoxic T cells (CTLs) against Tax (a major regulatory HTLV-1 protein) in IBMI-huNOG mice engrafted with CD133+ hSCs obtained from an HLA-A*24:02 cord blood donor. They show that the frequency of Tax301-309–specific CTLs among in vivo CD8+ T cells was inversely correlated with the PVL of infected cells. Second, the detection of immunoglobulin G antibodies against HTLV-1 antigens in the plasma of 2 infected mice is pleading for a class switching from IgM to IgG, thus supporting the existence of a functional interaction of human T and B cells. It should be emphasized that Tezuka et al are the first to report the development of functional HTLV-1 adaptive immune responses in humanized mice, suggesting that an adequate thymic education has occurred in IBMI-huNOG mice. As a matter of fact, humanized NSG-HLA-A2 mice (created by backcrossing the HLA class I transgene onto the NSG background) infected with EBV displayed human CTLs that recognized EBV-derived peptides in an HLA-restricted manner.9 Nevertheless, it is tempting to speculate that the detection of adaptive immune responses in IBMI-huNOG mice is linked to the use of CD133+ hSCs, to the engraftment procedure, and to the duration of the engraftment period. Indeed, CD133 has been identified as a reliable marker origin of human cord blood CD34– hSCs and is therefore considered as a specific marker of early hematopoietic progenitor cells.10 Furthermore, when directly injected into the bone marrow cavity, CD133+ hSCs are finding the right environment for their expansion and their differentiation into CD34+ cells, thus allowing the reconstitution of a functional human immune system.
Clearly, the generation of long-term engrafted immunocompetent mice, as reported by Tezuka et al, is opening a new chapter in the development of optimized humanized mice. Furthermore, their observations reinforce the use of humanized mouse models in investigating human viral pathogenesis. More specifically, this animal model raises high hopes for evaluating targeted therapies against ATL and for examining the role of stem cell transplantation in the treatment of ATL and other nonviral-induced leukemias.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal