In this issue of Blood, Tao et al provide a retrospective analysis of a large population-based data set to examine the relationships between socioeconomic status (SES), insurance status, demographic factors, and diffuse large B-cell lymphoma (DLBCL) disease–specific and overall survival. The authors address an important gap in the understanding of lymphoma disparities and take advantage of geospatial coded data within the California cancer registry that are appropriately suited to evaluate these relationships.1
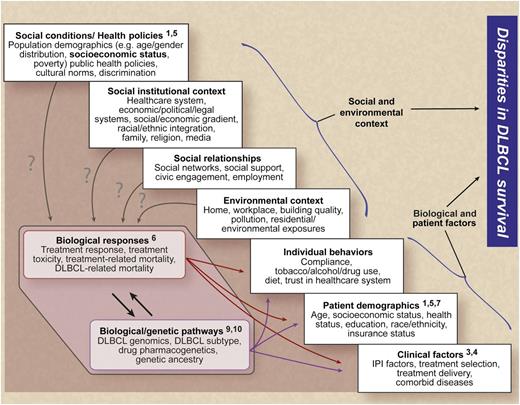
A framework for understanding the relationships between social, environmental, biological, and patient-related factors and disparities in DLBCL survival (numbers indicate example publications from the references that address specific factors). Professional illustration by Debra T. Dartez.
A framework for understanding the relationships between social, environmental, biological, and patient-related factors and disparities in DLBCL survival (numbers indicate example publications from the references that address specific factors). Professional illustration by Debra T. Dartez.
DLBCL is the most commonly occurring form of non-Hodgkin lymphoma in the Western world, comprising one-third of all lymphomas in adults. DLBCL represents a significant clinical problem for disparities research in that it is a curable disease, but one that is universally fatal if untreated or improperly treated. Untreated DLBCL patients have an expected survival of <1 year,2 whereas with modern chemoimmunotherapy 75% of patients achieve complete remission and 64% of these individuals are alive, disease-free at 10 years, and cured.3 Despite these high cure rates, DLBCL outcomes are heterogeneous and survival is lower for certain biological subtypes and patient subgroups.4-7
Tao and colleagues conducted a retrospective cohort analysis of 33 032 patients diagnosed with DLBCL in California from 1988 to 2009, and found that patients living in lower SES neighborhoods had increased risks of all-cause death and lymphoma-specific death when compared with patients in higher SES neighborhoods. These disparities suggest that socioeconomically disadvantaged patients experience barriers to effective DLBCL treatment including but not limited to inadequate insurance coverage. These findings are corroborated by a recent retrospective US study using the National Cancer Database, a nationwide, hospital-based cancer registry. That study demonstrated that among 3858 DLBCL patients who were 18 to 64 years of age, uninsured patients (hazard ratio [HR], 1.39; 95% confidence interval [CI], 1.14-1.70) and Medicaid-insured patients (HR, 1.48; 95% CI, 1.23-1.78) had lower survival than patients who had private insurance and that these associations were attenuated after adjustment for potential mediators such as failure to receive chemoimmunotherapy.5 Previous studies identified the relationships between race and/or lower SES and poorer DLBCL survival in the US population.5,7 However, no prior study considered how neighborhood SES influences racial/ethnic disparities in DLBCL. The authors present an attempt to disaggregate the relationships between these parameters and to examine their impact on DLBCL-specific survival. The study by Tao et al also aimed to understand whether socioeconomic disparities in DLBCL survival improved after advancements in DLBCL therapy.
This study reports that survival improved for all patients with DLBCL diagnosed from 2001 to 2009 compared with patients diagnosed from 1988 to 2000. These findings are consistent with other clinical and population-based studies reporting improvement in survival in the same era. An important finding in this study was the magnitude of disparity in survival associated with neighborhood SES which was more pronounced in the modern era (postrituximab adoption), in patients <65 years of age, and in married patients. Compared with patients living in the top 20% of neighborhoods ranked by SES, patients in the lowest 20% experienced 34% increased risk of death (all-cause) and 24% increased risk of DLBCL-specific death. Notable disparities by race/ethnic groups were delineated with higher rates of all-cause mortality experienced by Hispanics, Asian/Pacific Islanders, and African Americans relative to non-Hispanic whites after adjustment for age, sex, marital status, diagnostic period, stage, B symptoms, nodal status, treatment center, urbanicity, and first course of treatment. However, after further adjustment for neighborhood SES, all-cause mortality was similar across all racial/ethnic groups, suggesting that neighborhood SES disparities subsumed racial differences. In addition, uninsured and government-assisted insured patients experienced a 1.5-fold increased risk of death. The association of neighborhood SES and mortality risk was attenuated but not eliminated after adjusting for insurance status, suggesting an impact of SES beyond diminished access to care from being uninsured/underinsured. These findings highlight the association of SES and mortality risk in a curable disease, DLBCL, and suggest that barriers such as inadequate insurance coverage, financial burden, and access to effective therapy among disadvantaged patients exist. A significant limitation of this work that is common to all US-based registry studies is the lack of complete lymphoma treatment data. Nevertheless, the differences in DLBCL survival between the highest and lowest SES groups in this study are as large as what has been observed in randomized trials that changed therapy for DLBCL. What is more concerning about the authors’ findings is the fact that the relative disparities between groups increased following advances in therapy for DLBCL that improved survival for all patients.
This study identifies factors associated with disparities in survival and suggests that providing adequate insurance coverage without additional financial burden may reduce the widening disparities. However, eliminating disparities in DLBCL survival is multifaceted and must address complex associations between social, environmental, biological, and patient-centered factors. The figure utilizes a framework developed by the National Institutes of Health Centers for Population Health and Health Disparities8 to describe how these factors may interact to impact DLBCL survival. From a health policy perspective, changes in the US health care system may reduce the percentage of underinsured or uninsured. Examining practice patterns and health outcomes also may identify groups that fail to receive standard-of-care therapy and can inform public policy to improve access and care delivery. Although this study identifies an association of neighborhood SES and mortality, measuring the impact of living conditions, environmental exposures, and individual-level SES is needed to inform the task of eliminating disparities. At present, relatively little is known about the community infrastructure required to reduce barriers to care. For instance, transportation, sick pay, childcare, and how these affect health care access and outcomes have not been examined. Finally, the majority of biomedical research in DLBCL has focused on biological and patient-level factors that are known to influence DLBCL outcomes.6,9,10 Maximizing the impact of basic and translational research findings on DLBCL survival will require a more thorough understanding of the interactions between poor-risk biological, clinical, and socioeconomic groups. These interactions must be addressed to identify the most effective strategies for eliminating disparities in DLBCL outcomes.
Conflict-of-interest disclosure: C.R.F. has served as a consultant for OptumRx, Celgene, Seattle Genetics, Genentech/Roche (unpaid), and Millennium/Takeda (unpaid), and has received research funding from Abbvie, Celgene, Janssen, Millennium/Takeda, and Spectrum. L.J.N. has received consultation fees from Genentech. All funding for C.R.F. and L.J.N. has been for work performed outside of the current commentary.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal