Abstract
Paraneoplastic neurological syndromes (PNSs) rarely associate with Hodgkin lymphoma (HL) and non-HLs (NHLs). Except for paraneoplastic cerebellar degeneration (PCD) in HL and dermato/ polymyositis in both HL and NHL, other PNSs are uncommon and have only been reported as isolated case reports or short series. There are several important differences in PNSs when occurring in association with HL and NHL compared with those associated with solid tumors. First, some PNSs such as sensory neuronopathy or Lambert-Eaton myasthenic syndrome rarely occur in lymphomas, whereas others, such as granulomatous angiitis, are only described in HL. Second, onconeural antibodies are absent in most PNSs associated with lymphomas with the exceptions of Tr (δ/notch-like epidermal growth factor-related receptor) in PCD and mGluR5 in limbic encephalitis (LE). The antigens recognized by these antibodies are not expressed in lymphoma cells, suggesting the tumor itself does not trigger the PNS. Third, unlike patients with solid tumors in patients with lymphoma, the PNSs often develops at advanced stages of the disease. Furthermore, the type and frequency of PNSs are different between HL and NHL; whereas LE and PCD occur almost exclusively in patients with HL, sensorimotor neuropathies and dermatomyositis are more frequent in NHL.
Introduction
Paraneoplastic neurological syndromes (PNSs) occur with increased frequency in patients with cancer and are not caused by metastasis, direct infiltration of the tumor, or known indirect mechanisms such as toxicity, ectopic secretion of hormones, or induced coagulopathies. When originally described, the cause of PNSs was unknown. Presently, the accepted hypothesis is that many PNSs are caused by immune mechanisms triggered against antigens that are normally present in the nervous system and ectopically expressed by the tumor (onconeural antigens). The basis of this hypothesis is the identification of antibodies against onconeural antigens in serum and cerebral spinal fluid (CSF) of many patients with PNSs.1
The frequency of PNSs is low; they occur in <1% of patients with solid tumors, particularly small-cell lung carcinoma (SCLC), breast, and ovarian cancers. The frequency is probably lower in Hodgkin lymphoma (HL) and other lymphomas. However, the correct diagnosis of PNS is important because an early recognition of a neurological syndrome as paraneoplastic often leads to the discovery and treatment of the underlying tumor, which is a crucial step in the management of the PNS.1
Methods
References for this review were identified through searches of PubMed for articles published in English until December 31, 2013 with the search terms “Hodgkin disease,” “lymphoma,” in combination with “Ophelia syndrome,” “limbic encephalitis,” “granulomatous angiitis,” “paraneoplastic cerebellar degeneration,” “paraneoplastic chorea,” “opsoclonus,” “stiff-person syndrome,” “motor-neuron disease,” “paraneoplastic sensory neuropathy,” autoimmune autonomic neuropathy and/or ganglionopathy,” “paraneoplastic sensorimotor neuropathy,” “neuromyotonia,” “Lambert-Eaton myasthenic syndrome,” “polymyositis,” “dermatomyositis,” and “myasthenia.” Articles were also identified by searches of the authors’ files.
Diagnostic criteria of PNSs
The presence of a neurological syndrome of unclear etiology at the time of the diagnosis of a tumor does not necessarily mean that the neurological syndrome is paraneoplastic, as this could represent the coincidental occurrence of 2 unrelated events. In 2004, 2 levels of diagnostic certainty were proposed for PNSs: definite and possible. The criteria used to define the level are based on the type of neurological syndrome, the detection of well-characterized onconeural antibodies, and the presence of a cancer (Figure 1).2 Some PNSs are termed classical because they almost always indicate the presence of an underlying tumor (Table 1). These syndromes are considered definite PNSs if the tumor is found or the patient has a well-characterized onconeural antibody. Nonclassical syndromes, such as sensorimotor neuropathy, would qualify as a definite PNS only if the patient has a well-characterized onconeural antibody or the syndrome improves after successful treatment of the underlying tumor (Figure 1).2 Well-characterized onconeural antibodies are those that are demonstrated with validated tests, and for which there are a number of published reports defining the specificity and sensitivity of the antibody for PNS and confirmation of the findings by several investigators.2 Since the publication of the PNS criteria in 2004,2 2 onconeural antibodies should be added to the list of well-characterized onconeural antibodies: Sox1 antibodies which are markers of an underlying SCLC in patients with paraneoplastic cerebellar degeneration (PCD) or Lambert-Eaton myasthenic syndrome (LEMS),3,4 and Tr antibodies, which are markers of HL in patients with PCD.5 The Tr antigen has been recently identified as δ/notch-like epidermal growth factor-related receptor (DNER).6
Flowchart showing the level of diagnostic evidence for the diagnosis of PNSs. Reprinted with permission from J Neurol Neurosurg Psychiatry 2004;75:1135-1140.2
Flowchart showing the level of diagnostic evidence for the diagnosis of PNSs. Reprinted with permission from J Neurol Neurosurg Psychiatry 2004;75:1135-1140.2
Paraneoplastic neurological syndromes
| Syndrome . | Associated antibodies . | Predominant lymphoma type . | Selected references . |
|---|---|---|---|
| LE | mGluR5 | HL | 16 |
| Granulomatous angiitis | None | HL | 40, 41 |
| Cerebellar degeneration | Tr (DNER) | HL | 5, 6 |
| Paraneoplastic chorea | CV2/CRMP5* | <10 cases (NHL in 4) | 57, 58 |
| Opsoclonus-myoclonus | None | <10 cases (NHL in 3) | 64, 66 |
| Stiff-person syndrome | None | HL | 67, 72 |
| Paraneoplastic myelopathy | None | HL and NHL | 73, 74 |
| Motor neuronopathy | None | HL | 79, 80 |
| Sensory neuronopathy | None† | <10 cases (5 with HL) | 81, 82 |
| Autonomic ganglionopathy | nAChR‡ | <10 cases (HL, NHL) | 87, 88 |
| Sensorimotor neuropathy | None | HL and NHL | 94 |
| Vasculitic neuropathy | None | NHL | 97 |
| Neuromyotonia | None | <10 cases (HL, NHL) | 104 |
| Lambert-Eaton myasthenic syndrome | VGCC‡ | <10 cases (NHL) | 102 |
| Myasthenia | AChR‡ | HL and NHL | 105 |
| Dermatomyositis | p155 | NHL | 99 |
| Syndrome . | Associated antibodies . | Predominant lymphoma type . | Selected references . |
|---|---|---|---|
| LE | mGluR5 | HL | 16 |
| Granulomatous angiitis | None | HL | 40, 41 |
| Cerebellar degeneration | Tr (DNER) | HL | 5, 6 |
| Paraneoplastic chorea | CV2/CRMP5* | <10 cases (NHL in 4) | 57, 58 |
| Opsoclonus-myoclonus | None | <10 cases (NHL in 3) | 64, 66 |
| Stiff-person syndrome | None | HL | 67, 72 |
| Paraneoplastic myelopathy | None | HL and NHL | 73, 74 |
| Motor neuronopathy | None | HL | 79, 80 |
| Sensory neuronopathy | None† | <10 cases (5 with HL) | 81, 82 |
| Autonomic ganglionopathy | nAChR‡ | <10 cases (HL, NHL) | 87, 88 |
| Sensorimotor neuropathy | None | HL and NHL | 94 |
| Vasculitic neuropathy | None | NHL | 97 |
| Neuromyotonia | None | <10 cases (HL, NHL) | 104 |
| Lambert-Eaton myasthenic syndrome | VGCC‡ | <10 cases (NHL) | 102 |
| Myasthenia | AChR‡ | HL and NHL | 105 |
| Dermatomyositis | p155 | NHL | 99 |
Classical syndromes are underlined. nAChR, nicotinic acetylcholine receptor; VGCC, voltage-gated calcium channel.
Not present in all cases.
One patient with NHL and Ma2 antibodies (not published).
Marker of the syndrome, not predictor of cancer.
The recently described antibodies against neuronal cell surface or synaptic receptors are not included in the group of well-characterized onconeural antibodies because they are excellent markers (and appear to be pathogenic) of the neurological syndrome, but may occur in patients with or without cancer (Table 2).7-16 For these cell surface or synaptic antibodies, the frequency of an underlying tumor varies with the type of antibody, age, and sometimes gender of the patient; in some cases (eg, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] or γ-aminobutyric acid B [GABAB] receptor antibodies), a search for an underlying tumor is indicated because cancer may be present in up to 60% of the patients (Table 2).7
Antibodies against cell surface or synaptic antigens
| Antibody . | Syndrome . | Cancer . |
|---|---|---|
| NMDAR | Encephalitis | Ovarian teratoma (rare in children, present in 58% older than 18 y)8 |
| LGI1 | LE | None9 |
| VGCC | LEMS | SCLC (50%)10 |
| GABABR | LE | SCLC (70%)11 |
| CASPR2 | Morvan syndrome | Thymoma (38%)12 |
| AMPAR | LE | SCLC, breast, thymus (60%)13 |
| DPPX | Encephalitis | None14 |
| mGluR1 | Cerebellar ataxia | A few cases reported, two with HL15 |
| mGluR5 | LE | HL (only two cases reported)16 |
| Antibody . | Syndrome . | Cancer . |
|---|---|---|
| NMDAR | Encephalitis | Ovarian teratoma (rare in children, present in 58% older than 18 y)8 |
| LGI1 | LE | None9 |
| VGCC | LEMS | SCLC (50%)10 |
| GABABR | LE | SCLC (70%)11 |
| CASPR2 | Morvan syndrome | Thymoma (38%)12 |
| AMPAR | LE | SCLC, breast, thymus (60%)13 |
| DPPX | Encephalitis | None14 |
| mGluR1 | Cerebellar ataxia | A few cases reported, two with HL15 |
| mGluR5 | LE | HL (only two cases reported)16 |
AMPAR, AMPA receptor; CASPR2, contactin-associated protein 2; DPPX, dipeptidyl-peptidase-like protein-6; GABABR, GABAB receptor; LGI1, leucine-rich, glioma inactivated 1 protein; NMDAR, N-methyl-d-aspartate receptor; SCLC, small-cell lung cancer; VGCC, voltage-gated calcium channel.
General overview of PNSs in lymphomas
PNSs are rare in lymphomas. Except for PCD in HL and dermato/ polymyositis in both HL and non-HL (NHL), other PNSs have rarely been reported in these patients. The frequency of PNSs associated with lymphomas was analyzed by the PNS Euronetwork consortium that includes 20 European centers. Between 2000 and 2008, the consortium collected 53 patients with PNSs, 29 of them with NHL and 24 with HL. The most commonly associated PNS was PCD in 21 patients, 16 of them with HL, and demyelinating neuropathies in 11, 9 with NHL.17
PNSs that occur in lymphomas have several important differences compared with those in solid tumors. First, lymphomas associate with unique PNSs (granulomatous angiitis, hypothermia), whereas classical PNSs (sensory neuronopathy, LEMS) rarely occur. Second, onconeural antibodies are absent in most PNSs (Table 1); only Tr and metabotropic glutamate receptor type 5 (mGluR5) are considered good markers of an underlying HL. This is a limitation to define as definite many PNS that associate with lymphomas. Third, onconeural antigens are not expressed in the tumor cells, suggesting the tumor itself may not trigger the PNS. Fourth, unlike solid tumors, lymphomas of patients with PNSs do not usually have limited extension at the time of diagnosis. In Table 1 and the text, we summarize the PNSs that have been described in HL and NHL. The type and frequency of PNSs is different between HL and NHL. The global incidence of PNSs is probably higher in HL. Limbic encephalitis (LE) and PCD are almost exclusively seen in patients with HL, whereas sensorimotor neuropathies and dermatomyositis are more common in NHL.
LE
Patients with LE develop short-term memory loss or amnesia, disorientation, confusion, depression, anxiety, or frank psychosis with visual or auditory hallucinations or paranoid ideation. Generalized or partial complex seizures occur in ∼50% of the patients. HL is the third most common cause of LE after SCLC and testicular germ cell tumors.18 The cases reported in the English literature are summarized in Table 3.16,18-29 The association of LE with HL is also known as Ophelia syndrome.19 The clinical and magnetic resonance imaging (MRI) features (Figure 2) are not different from those reported in LE associated with other tumors. However, the LE of patients with HL has a better prognosis; frequently, successful treatment of the tumor is sufficient to result in full neurological recovery. This is probably related to the finding that this type of LE occurs in association with an antibody against the mGluR5 that probably results in reversible neuronal dysfunction rather than neuronal death (Figure 3).16,30
LE and HL
| Reference . | Age (y)/gender . | Delay* (months) . | Type (stage) . | Antibody . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|
| 18 | 15/F | 4-6 | Unknown | ND | Oncological | Full recovery |
| 19 | 36/M | 9 | NS (IV) | ND | Oncological, steroids | No improvement |
| 20 | 33/M | 0 | NS (IV) | ND | Oncological | Full recovery |
| 21 | 23/M | 10 | NS (III) | ND | Steroids,† Oncological | Full recovery |
| 22 | 13/F | ? | NS (?) | ND | Oncological | No improvement |
| 23 | 53/F | 6 | NS (II) | Negative‡ | Oncological | Full recovery |
| 24 | 42/M | 52 | MC (II) | ND | Oncological | No improvement |
| 25 | 69/F | 6 | MC (II) | Negative‡ | Oncological | Unknown |
| 26 | 61/M | 0 | MC (III) | Hu‡ | Oncological | Full recovery |
| 27 | 49/M | 0§ | NS (?) | NMDAR | Steroids, IVIG, PLEX, oncological | Partial improvement |
| 16 | 46/F | 0 | ? (III) | mGluR5 | Steroids, oncological | Full recovery |
| 28 | 62/F | ? | ? (I) | Hu‡,¶ | None | Full recovery |
| 29 | 35/M | 6 | NS (II) | mGluR5 | Oncological | Full recovery |
| Reference . | Age (y)/gender . | Delay* (months) . | Type (stage) . | Antibody . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|
| 18 | 15/F | 4-6 | Unknown | ND | Oncological | Full recovery |
| 19 | 36/M | 9 | NS (IV) | ND | Oncological, steroids | No improvement |
| 20 | 33/M | 0 | NS (IV) | ND | Oncological | Full recovery |
| 21 | 23/M | 10 | NS (III) | ND | Steroids,† Oncological | Full recovery |
| 22 | 13/F | ? | NS (?) | ND | Oncological | No improvement |
| 23 | 53/F | 6 | NS (II) | Negative‡ | Oncological | Full recovery |
| 24 | 42/M | 52 | MC (II) | ND | Oncological | No improvement |
| 25 | 69/F | 6 | MC (II) | Negative‡ | Oncological | Unknown |
| 26 | 61/M | 0 | MC (III) | Hu‡ | Oncological | Full recovery |
| 27 | 49/M | 0§ | NS (?) | NMDAR | Steroids, IVIG, PLEX, oncological | Partial improvement |
| 16 | 46/F | 0 | ? (III) | mGluR5 | Steroids, oncological | Full recovery |
| 28 | 62/F | ? | ? (I) | Hu‡,¶ | None | Full recovery |
| 29 | 35/M | 6 | NS (II) | mGluR5 | Oncological | Full recovery |
IVIG, intravenous immunoglobulins; MC, mixed cellularity; ND, not done; NS, nodular sclerosis; PLEX, plasma exchange.
Delay between onset of LE and HL diagnosis.
No improvement.
Antibodies to surface antigens were not tested.
At relapse.
CSF negative.
Coronal T2-weighted MRI scan of a patient with LE, mGluR5 antibodies, and HL. There is increased T2 signal of the head of both hippocampi. Study is slightly affected by motion artifact.
Coronal T2-weighted MRI scan of a patient with LE, mGluR5 antibodies, and HL. There is increased T2 signal of the head of both hippocampi. Study is slightly affected by motion artifact.
Immunoreactivity of mGluR5 antibodies. (A) Sagittal section of rat brain immunostained with the CSF of a patient with mGluR5 antibodies. There is a diffuse staining of the neuropil. (B) Immunoreactivity was particularly robust in the hippocampus that shows the typical staining of antibodies against neuronal cell surface antigens.
Immunoreactivity of mGluR5 antibodies. (A) Sagittal section of rat brain immunostained with the CSF of a patient with mGluR5 antibodies. There is a diffuse staining of the neuropil. (B) Immunoreactivity was particularly robust in the hippocampus that shows the typical staining of antibodies against neuronal cell surface antigens.
The diagnosis of LE requires electroencephalographic, neuroradiological, or pathological evidence of involvement of the medial temporal lobes and amygdala.18 It is important to apply these criteria to avoid labeling, as LE, patients with other disorders that may also associate with HL.31 The application of these criteria revealed 2 patients, 14 and 18 years of age, reported as LE who in fact probably had other forms of encephalitis. Both cases presented with fever, headache, nausea, and vomiting, and CSF pleocytosis followed in a few days by progressive confusion, agitation, hallucinations (1 patient), and seizures, requiring intubation. Brain MRI was initially normal, and both patients made excellent recovery after treatment of the HL.32,33
Unlike HL, LE is extremely rare in patients with NHL, with only a few cases described, mostly in the Japanese literature.34,35 As in some HL cases, the criteria for LE were not fulfilled in 2 patients.36,37 In a third patient, the syndrome attributed to LE occurred after an allogeneic bone marrow transplant, raising the concern for a possible infection by herpes virus type 6.38,39
Granulomatous angiitis of the central nervous system
This disorder is histopathologically characterized by the presence of necrotizing vasculitis involving small arteries and veins along with noninfectious granulomas composed of lymphocytes, monocytes, and plasma cells. Leptomeningeal vessels are preferentially affected. The cause of this vasculitis is unknown, but there is evidence that it could be related to the varicella-zoster virus.40 The disorder may precede or occur shortly after the diagnosis of HL. In the majority of cases, the vasculitis has not been linked to a recent infection of varicella-zoster, and the pathological studies ruled out the presence of viral particles in the affected vessel walls, supporting a possible autoimmune mechanism.41 Table 441-46 summarizes the cases described since 2000 with a confirmed pathological diagnosis (previous cases are reviewed in ref. 42).
Pathologically (biopsy or autopsy) confirmed cases of granulomatous angiitis of the CNS and HL since the year 2000
| Reference . | Age (y)/gender . | Presentation . | No. WBC/µL in CSF . | MRI . | Outcome . |
|---|---|---|---|---|---|
| 42 | 27/F | Headache | Not done | Gd-enhancing lesion | Full recovery |
| 43 | 52/M | Seizures, confusion | Not specified | Diffuse leukoencephalopathy | Full recovery |
| 41 | 26/F | Headache, seizures | 55 | Gd-enhancing lesions | Full recovery |
| 44 | 16/M | Aphasia, vomiting | 15 | Hemorrhage | Full recovery |
| 45 | 37/M | Gait ataxia | 42 | Diffuse Gd-enhancing lesions | Unknown |
| 46 | 58/M | Left hemiparesis | 15 | Bilateral leukoencephalopathy | Full recovery |
| Reference . | Age (y)/gender . | Presentation . | No. WBC/µL in CSF . | MRI . | Outcome . |
|---|---|---|---|---|---|
| 42 | 27/F | Headache | Not done | Gd-enhancing lesion | Full recovery |
| 43 | 52/M | Seizures, confusion | Not specified | Diffuse leukoencephalopathy | Full recovery |
| 41 | 26/F | Headache, seizures | 55 | Gd-enhancing lesions | Full recovery |
| 44 | 16/M | Aphasia, vomiting | 15 | Hemorrhage | Full recovery |
| 45 | 37/M | Gait ataxia | 42 | Diffuse Gd-enhancing lesions | Unknown |
| 46 | 58/M | Left hemiparesis | 15 | Bilateral leukoencephalopathy | Full recovery |
Gd, gadolinium; WBC, white blood cells.
Patients develop headache, subacute cognitive decline or confusion, and focal neurological symptoms. The CSF analysis shows lymphocytic pleocytosis. Brain MRI may demonstrate bilateral areas of leukoencephalopathy, gadolinium-enhancing lesions that follow a perivascular pattern, or, less frequently, hemorrhagic lesions.41 Cerebral angiography may show small vessel beading, suggestive of vasculitis, but in general, it has low sensitivity.40 Unlike patients reported in previous decades, recent cases of granulomatous angiitis of the central nervous system (CNS) and HL had full recovery after treatment with steroids and chemotherapy for the underlying HL (Table 4).
PCD
PCD is one of the best-characterized PNS and occurs preferentially associated with ovarian, breast, SCLC or HL.47 Only a few cases of PCD associated with NHL have been reported.17,48-50 The pathological hallmark of PCD is a severe loss of Purkinje cells of the cerebellum that results in subacute pancerebellar dysfunction. The usual presentation is dizziness and vertigo that rapidly progress to severe usually symmetrical truncal and limb ataxia, with dysarthria, diplopia, and frequent down-beat nystagmus. CSF examination usually shows moderate pleocytosis, and the MRI studies are initially normal and later show cerebellar atrophy.1
PCD antedates the diagnosis of HL in 80% of the patients.5 The most useful diagnostic test is the detection of onconeural antibodies. Patients with PCD and HL may harbor Tr antibodies (named after the author that first identified them51 ) in their serum and CSF (Figure 4).52 The target antigen of these antibodies is DNER,6 a transmembrane protein that is preferentially expressed in the dendrites of Purkinje cells and likely plays a regulatory role in dendrite patterning.53 Unlike other PNS antigens, DNER has not been identified in tissue samples of HL, suggesting that the immune response is not triggered by ectopic tumor expression of the antigen.6 Antibodies against the mGluR1 were initially described in 2 patients who developed subacute cerebellar ataxia 2 and 9 years after HL. Subsequent case reports have not confirmed the association of PCD and mGluR1 antibodies with HL.54,55
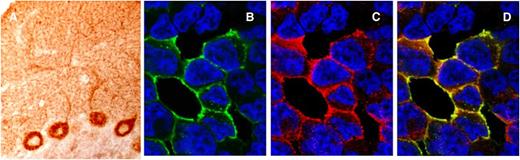
Immunoreactivity of Tr(DNER) antibodies. (A) Section of rat cerebellum immunostained with a serum from a patient with Tr antibodies. There is robust staining of the cytoplasm and the main dendrites of the Purkinje cells and a dot-like pattern of staining of the cerebellar molecular layer that is characteristic of Tr immunoreactivity. (B-D) Cell-based assay to confirm that Tr antibodies identify the DNER antigen. HEK293 cells transfected with DNER cDNA plasmid show intense reactivity with the human serum positive for Tr antibodies (green) and that colocalizes (yellow) with the labeling of a commercial monoclonal antibody to DNER (red).
Immunoreactivity of Tr(DNER) antibodies. (A) Section of rat cerebellum immunostained with a serum from a patient with Tr antibodies. There is robust staining of the cytoplasm and the main dendrites of the Purkinje cells and a dot-like pattern of staining of the cerebellar molecular layer that is characteristic of Tr immunoreactivity. (B-D) Cell-based assay to confirm that Tr antibodies identify the DNER antigen. HEK293 cells transfected with DNER cDNA plasmid show intense reactivity with the human serum positive for Tr antibodies (green) and that colocalizes (yellow) with the labeling of a commercial monoclonal antibody to DNER (red).
Although DNER is a cell surface antigen, PCD related to antibodies against this antigen does not respond as well to treatment as other disorders associated to antibodies against cell surface antigens (Table 2). However, the prognosis is still better than that of PCD associated with solid tumors.47 In the 2 largest series of PCD and HL with a total of 49 patients, 7 (14%) had full recovery or partial neurological improvement.5,56 Patients <40 years of age were more likely to improve, and a few had spontaneous improvement. The Tr (or DNER) antibody titers usually disappear after treatment of the HL.5
Paraneoplastic chorea
A paraneoplastic etiology is rarely considered in the differential diagnosis of chorea. However, 56 well-documented cases have been reported, 4 of them associated with NHL and 1 with HL.57,58 The chorea was generalized and associated with peripheral neuropathy or encephalopathy in 2 patients. Brain MRI may demonstrate bilateral hyperintensity of caudate nuclei and putamen, as can be seen in paraneoplastic chorea associated with other tumors, mainly SCLC.59 The most useful diagnostic test is determination of CV2/collapsin response mediator protein 5 (CRMP5) antibodies, which are often detected in patients with paraneoplastic chorea regardless of the tumor association.60 However, almost 50% of patients with paraneoplastic chorea with solid tumors or lymphomas do not have onconeural antibodies. Neurological improvement may occur after immunotherapy or treatment of the lymphoma but a complete remission is rarely achieved.57
Opsoclonus-myoclonus syndrome
Opsoclonus is defined by irregular, continuous, large amplitude conjugate saccades in all directions of gaze. In opsoclonus-myoclonus syndrome, the abnormal eye movements are associated with arrhythmic action myoclonus that involves trunk, limbs, and head, along with truncal ataxia, dysarthria, and, in some patients, confusion and coma. Opsoclonus may result from a wide variety of causes, the most frequent being postinfectious, idiopathic, and paraneoplastic.61 In children, paraneoplastic opsoclonus-myoclonus occurs in association with neuroblastoma. In adults, the syndrome is mainly associated with breast cancer and gynecologic tumors (usually accompanied by the presence of Ri antibodies) and SCLC (usually without paraneoplastic antibodies),62 Only 4 patients with this syndrome and NHL or HL have been reported. None of the 3 patients with NHL improved63-65; the patient with HL developed opsoclonus-myoclonus 7 weeks after autologous hematopoietic cell transplantation, and therefore a postinfectious etiology could have been the cause of the disorder.66
Paraneoplastic stiff-person and related syndromes
Stiff-person syndrome is a rare CNS disorder characterized by progressive muscular rigidity, predominantly of the trunk with superimposed spasms. The diagnosis is based on established clinical and neurophysiological findings, including continuous co-contraction of agonist and antagonist muscles caused by involuntary firing of motor units at rest. Many cases of stiff-person syndrome associate with glutamic acid decarboxylase antibodies.67 A related syndrome, progressive encephalomyelitis with rigidity and myoclonus (PERM), is characterized by subacute onset of muscular rigidity, stimulus-sensitive spasms, brainstem dysfunction, and pathological findings of perivascular lymphocyte cuffing and neuronal loss in the brainstem and spinal cord.68 It is unclear if PERM is a completely different disease from stiff-person syndrome or a particularly severe form of the disorder. Recently, glycine receptor antibodies were described in some patients with PERM; these antibodies have also been described in some patients with stiff-person syndrome.69 Less than 1% of cases with stiff-person syndrome are paraneoplastic, and some with breast cancer or SCLC associate with amphiphysin antibodies. This syndrome has been reported in 5 patients with HL. None of them presented known antibodies, but the neurological symptoms recovered when the HL was successfully treated, suggesting the syndrome could have been mediated by antibodies against surface antigens.67,70-72
Paraneoplastic myelopathy
Paraneoplastic involvement of the spinal cord presents with subacute myelitis and rapidly ascending paralysis accompanied by a sensory level.73 The syndrome has been described in patients with solid tumors and lymphomas. In the latter, the possibility of a toxic or infectious origin was not completely ruled out.74 The initial patients reported developed a severe and irreversible paraplegia, and postmortem studies of 2 cases revealed widespread spinal cord necrosis without prominent inflammatory infiltrates or vasculitis (necrotizing myelopathy).74 This type of myelopathy has been described in a few cases with HL and NHL.75,76 In the last decade, patients reported with paraneoplastic myelopathies have had better outcomes, and in 1 study, 31% of cases improved with treatment.73 The MRI usually shows a longitudinally extensive (>3 vertebral segments) T2 increased signal abnormality that may enhance with gadolinium. Onconeural antibodies (mainly amphiphysin and CV2/CRMP5 antibodies) may be present in serum or CSF, supporting the paraneoplastic origin of the myelitis. However, patients with HL or NHL can be seronegative, and some cases were not tested for onconeural antibodies.73,77
Paraneoplastic neuronopathies
The term neuronopathy indicates that the primary damage occurs in the cell body of neurons that form the peripheral nerves. There are PNSs that target a specific neuronal type and cause pure motor, sensory, or autonomic neuronopathies and others that damage more than cell type, leading to a mixed neuronopathy. The latter situation is more frequent in the setting of SCLC and Hu antibodies.78 Paraneoplastic motor neuronopathies have been described in a few patients who almost always had HL. The syndrome defined as subacute motor neuropathy has only rarely been reported since the initial description in 1979.79 Patients develop motor weakness that tends to be greater in the legs and proximal muscles. Muscle weakness is rarely severe and sometimes stabilizes or improves without any specific treatment. The symptoms appear when the HL is already diagnosed or in remission. Some patients developed the disorder after treatment with mantle and para-aortic radiotherapy, and radiation toxicity could not be completely ruled out.79 In other cases, the patients had more severe and diffuse weakness antedating the diagnosis of HL. Although some pathological reports show loss of motor neurons in the anterior horn of the spinal cord, the clinical improvement in some patients suggest the presence of mechanisms affecting neuronal function rather than causing irreversible degeneration.80
Sensory neuronopathy is caused by damage of sensory neurons in the dorsal root ganglia and is one of the most common and well-characterized PNSs, usually occurring in association with SCLC and Hu antibodies.78 The syndrome typically runs a subacute clinical course with pain and paresthesias in the upper or lower limbs. The presenting symptoms are usually asymmetric or affect only 1 extremity. Neurological examination reveals involvement of all modalities of sensation with severe impairment of joint position and vibratory senses. Deep tendon reflexes are abolished or diminished. Electromyography and nerve conduction velocities are helpful in confirming that the involvement is restricted to the sensory nerves. The prognosis is poor and patients rarely improve.
Paraneoplastic sensory neuronopathy has only been reported in a few patients mainly with HL; onconeuronal antibodies are negative.81-84 However, we evaluated a patient with this neuropathy and NHL who had Ma2 antibodies (F. Graus, 2013, unpublished data). There are also a few case reports of patients with a subacute sensory neuropathy that had complete recovery after treatment of the HL. These cases probably represent sensory variants of inflammatory demyelinating neuropathies rather than true neuronopathies.85
When the neuronal bodies of autonomic ganglia are affected, patients develop subacute pandysautonomia with impairment of sympathetic (orthostatic hypotension, anhydrosis) and parasympathetic function (impaired pupillary responses, dry mouth, gastrointestinal dysmotility, erectile dysfunction, and fixed heart rate).86 The syndrome rarely has a paraneoplastic origin and is frequently associated with nicotinic acetylcholine receptor antibodies. The presence of these antibodies helps to confirm the neurological diagnosis but does not establish a paraneoplastic etiology.86 The association of autoimmune autonomic ganglionopathy with lymphomas is exceptional.87,88 One of the reported cases improved after treatment with rituximab.88 A limited form of autonomic ganglionopathy is the occurrence of an isolated tonic (Adie) pupil that has been described in patients with cancer, including one with HL and Tr (DNER) antibodies.89
An intriguing symptom identified in a few patients with HL is hypothermia. Severe episodes of hypothermia were reported in 17 patients with advanced stage HL usually during chemotherapy. The cause of these episodes is unknown.90
Paraneoplastic sensorimotor neuropathies
Paraneoplastic sensorimotor neuropathies that precede the diagnosis of lymphomas are mainly demyelinating neuropathies that may fulfill diagnostic criteria of Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy (CIDP).91 The clinical course is usually subacute, and onconeural antibodies are negative. Paraneoplastic neuropathies are more common in HL and NHL with monoclonal bands. Although the frequency of cancer in patients with Guillain-Barré syndrome is not increased, the association between this neuropathy and HL is well established.92 Patients with NHL and monoclonal gammopathy can develop typical CIDP or predominantly sensory neuropathies that are likely mediated by antibody activity of the monoclonal IgM against myelin-associated glycoprotein or gangliosides.93,94 Conversely, neuropathies associated with large B-cell lymphomas may be caused by direct infiltration of the peripheral nerves (neurolymphomatosis).95 In a recent study of 32 patients with neuropathy and NHL, 15 were considered to have neurolymphomatosis, whereas only 5 were paraneoplastic. In the other 12 patients, the etiology was uncertain, and all but 2 developed a syndrome of multifocal mononeuropathy suggestive of neurolymphomatosis.94 Although infiltrative neuropathies usually are asymmetric or show a pattern of multifocal mononeuropathy, they can be indistinguishable from CIDP and even respond to steroids.94 The diagnosis is usually confirmed by the demonstration of malignant cells in the nerve biopsy or more rarely in the CSF (Figure 5). Fluorodeoxyglucose positron emission tomography/computed tomography may help in identifying abnormal areas in peripheral nerves or plexuses susceptible to biopsy.94
Sural nerve biopsy of a patient with a final diagnosis of neurolymphomatosis. (A) Low power figure of the sural nerve biopsy showing infiltration by lymphomatous cells. (B) The infiltrate involves all layers of the nerve. Arrowheads indicate the perineurium.
Sural nerve biopsy of a patient with a final diagnosis of neurolymphomatosis. (A) Low power figure of the sural nerve biopsy showing infiltration by lymphomatous cells. (B) The infiltrate involves all layers of the nerve. Arrowheads indicate the perineurium.
Vasculitic neuropathy
Isolated vasculitis of peripheral nerves has been reported in a few patients with solid tumors and lymphomas.96 The clinical course is characterized by a progressive, initially asymmetric, painful sensorimotor neuropathy. Electromyography and nerve conduction studies demonstrate findings compatible with multifocal neuropathy or diffuse axonal polyneuropathy with asymmetric involvement.97 Combined biopsy of nerve and muscle is indicated because this increases the chance of identifying the vasculitis. Nerve biopsy shows intramural and perivascular inflammatory infiltrates, usually without necrotizing vasculitis.98
Paraneoplastic syndromes of the neuromuscular junction and muscle
The most frequent PNS of the muscle in patients with lymphoma is dermatomyositis.99 Some patients with lymphoma develop polymyositis, but the frequency of this association is much lower than that with dermatomyositis. Cancer-associated myositis (dermato- and polymyositis) occurs in patients >50 years of age and is more predominant in NHL.99 In ∼50% of patients, the myositis is diagnosed before the discovery of the lymphoma. Thus, a search for an underlying cancer is indicated in these patients, and whole body positron emission tomography/computed tomography is the preferred test.100 Immunological studies may also help in identifying patients at risk of developing cancer. The presence of anti-synthetase antibodies is a negative predictor for the presence of cancer. In contrast, detection of the antibody p155, against human transcriptional intermediary factor 1-γ, shows a sensitivity of 70% and a specificity of 89% for predicting an occult malignancy. The data are more robust for patients with dermatomyositis, with only 5% of p155 antibody-negative patients subsequently developing cancer.101
Lymphomas are a rare cause of PNSs of the neuromuscular junction. Only a few patients, usually with NHL and LEMS102 or neuromyotonia, have been reported.103,104 A few cases of myasthenia gravis have been described in association with HL and T-cell NHL with involvement of the mediastinum.105 In 50% of the cases with lymphoma and LEMS or myasthenia, the neurological disorder develops around the time of the diagnosis of lymphoma. For the rest of patients, the lymphoma and neurological disorder are separated by many years, suggesting the presence of an underlying immunological disturbance caused by the tumor or immunosuppression that predisposes to the paraneoplastic immune-mediated syndrome.
Acknowledgments
The authors thank Dr Josep Maria Grau for providing the pathological specimen of Figure 5.
This work was supported in part by grants 11/01780 (to J.D.) and PI12/00611 (to F.G.) from the Fondo Investigaciones Sanitarias, Fundació la Marató TV3 (J.D.) and National Institutes of Health, National Institute of Neurological Disorders and Stroke grant RO1NS077851 (to J.D.).
Authorship
Contribution: F.G. participated in the literature search, creating figures, study design, data collection, data analysis, data interpretation, writing, and critical approval of the final manuscript; H.A. participated in data collection, data interpretation, critical approval of the final manuscript, and funding; and J.D. participated in creating figures, study design, data collection, data analysis, data interpretation, writing, and critical approval of the final manuscript.
Conflict-of-interest disclosure: J.D. receives royalties from licensing fees from Euroimmun for a patent for the use of NMDAR as autoantibody test. The remaining authors declare no competing financial interests.
Correspondence: Francesc Graus, IDIBAPS-Hospital Clínic, Universitat de Barcelona, Department of Neurology, Villarroel 170, Barcelona 08036, Spain; e-mail: fgraus@clinic.ub.es.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal