In this issue of Blood, Duckworth et al find that in chronic lymphocytic leukemia (CLL), malignant cell anergy is associated with failure of inducing PRDM1 (BLIMP1), a critical regulator of differentiation into plasma cells, and that epigenetic modifications account for such failure. These findings link two major problems of CLL cells, the anergic response to B-cell receptor (BCR) stimulation and the incapacity to differentiate.1
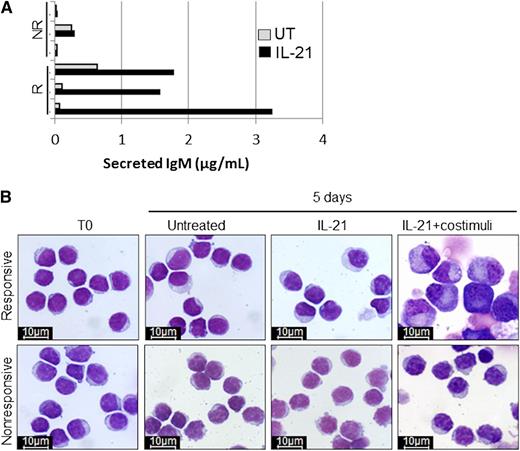
Immunoglobulin M (IgM) secretion and cell morphology after 5 days of in vitro treatment of responsive (R) and non-responsive (NR) CLL samples with IL-21. See Figure 4A-B in the article by Duckworth et al that begins on page 3277.
Immunoglobulin M (IgM) secretion and cell morphology after 5 days of in vitro treatment of responsive (R) and non-responsive (NR) CLL samples with IL-21. See Figure 4A-B in the article by Duckworth et al that begins on page 3277.
In normal B cells, the engagement of BCR induces either proliferation and then differentiation into antibody-producing cells or a reversible lethargic state named anergy, a sort of limbo that B cells enter when they encounter an antigen (usually an autoantigen), in the absence of the costimulation provided by T cells.2 Because the engagement of BCR has a key role in the pathogenesis of CLL,3 the question becomes if and how CLL leukemic cells differ from normal B cells when their BCR is stimulated. There are two main differences. First, the proliferating CLL B cell fails to undergo plasma cell differentiation. Hence, no antibody is produced to neutralize the triggering antigen whose stimulating activity may proceed unabated and favors clonal expansion. Second, although normal anergic B cells are short-lived and prone to apoptosis (thus preventing the development of dangerous autoreactive cells),4 CLL cells are not, as they are uniformly protected by the overexpression of the antiapoptotic protein BCL2. Duckworth et al,1 using different stimuli such as interleukin-21 (IL-21) and cytosine guanine dinucleotide-oligodeoxynucleotides that robustly induce differentiation into plasma cells and having the expression of PRDM1 as readout are able to show that: (1) the reduced differentiation capacity of anergic CLL cells is independent of the signaling pathway; (2) at variance with normal B cells the costimulation of anergic CLL cells does not overcome the differentiation hurdle; and (3) the reduced capacity of inducing PRDM1 is also reflected by a block in immunoglobulin secretion (see figure). Of interest, they also observe the reversing of CLL cell anergy by appropriate in vitro culture associated with the ability to induce expression of PRDM1 upon adequate stimuli.
BCR-induced cell proliferation tends to be associated with unmutated (U) IgVH gene status and anergy with mutated (M) IgVH gene status.3 Anergic CLL B cells can be identified by their molecular signature.5 Considering that the mutational status of IgVH genes is an important prognostic determinant with U-CLL bearing a worse prognosis, the modality of response to BCR stimulation with the sequence Ag stimulation→proliferation is considered dangerous, whereas the sequence Ag stimulation→anergy is regarded as more advantageous and has been taken to partly explain the more indolent clinical behavior of M-CLL. However, even if anergy is more evident in M-CLL, it is also present in U-CLL, and in both types of CLL the critical point may be represented by the balance between cell lethargy and cell proliferation.3 It is plausible to postulate that anergy is reverted and proliferation starts when antigen-triggered CLL B cells encounter T cells. This encounter likely occurs within the tissue microenvironment, especially in the proliferative clusters known as pseudofollicles (and perhaps might even give rise to their development).6 As most CLL studies have been performed using cells from peripheral blood, the results may be a pale reflection of the events that occur in the tissues where the proliferation rate has been found to be much higher than anticipated.7,8 As anergic cells can be recruited in the proliferation process both in M- and in U-CLL, they might represent a major clonal reservoir of indecisive, hesitant cells that are persuaded to proliferate by the proper T-cell encounter. The pool of anergic cells likely gives shelter to subclones harboring dangerous mutations, and these subclones may be expanded by subsequent rounds of proliferation.
The implication is that CLL anergic cells are a potentially important therapeutic target. When the anergic state has been experimentally reverted, the leukemic cells were found to undergo apoptosis.9 This brings in another interesting finding of this paper. By investigating both DNA methylation and histone modifications associated with PRDM1 transcriptional control elements, the authors find that the transcriptional inactivity of the PRDM1 gene is due to transcriptional repression and failure to facilitate gene transcription.1 The observation that anergy and the differentiation block are linked by epigenetic modifications may open a novel therapeutic avenue. Conceivably, the anergic state might be reverted by means of epigenetic-interfering drugs. This leads us to envisage the possibility of a combination treatment policy, whereby epigenetic-interfering or other drugs able to modify the anergic status might be added to those that efficaciously target BCR signaling.10
Conflict-of-interest disclosure: The author declares no competing financial interests.