In this issue of Blood, Kasirer-Friede and colleagues show that the adhesion and degranulation promoter protein (ADAP) promotes αIIbβ3 activation by presenting the cytoplasmic proteins talin and kindlin to the β3 cytoplasmic tail.1
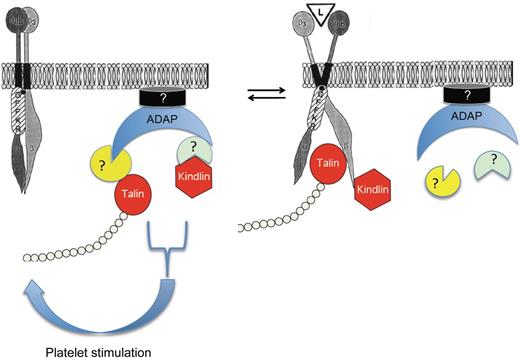
Talin and kindlin bind to ADAP in resting platelets and are transferred to the β3 cytoplasmic tail after platelet stimulation. Whether intermediary proteins are involved in the associations of ADAP with the platelet membrane and with talin and kindlin is not clear. L, αIIbβ3 ligand; GFFKR, conserved membrane proximal region of the αIIb cytoplasmic tail that helps to maintain inactive αIIbβ3. Adapted with permission from Figure 8 in O'Toole et al.11 © 1994 Rockefeller University Press. Originally published in Journal of Cell Biology. 124:1047-1059. doi:10.1083jcb.124.6.1047.
Talin and kindlin bind to ADAP in resting platelets and are transferred to the β3 cytoplasmic tail after platelet stimulation. Whether intermediary proteins are involved in the associations of ADAP with the platelet membrane and with talin and kindlin is not clear. L, αIIbβ3 ligand; GFFKR, conserved membrane proximal region of the αIIb cytoplasmic tail that helps to maintain inactive αIIbβ3. Adapted with permission from Figure 8 in O'Toole et al.11 © 1994 Rockefeller University Press. Originally published in Journal of Cell Biology. 124:1047-1059. doi:10.1083jcb.124.6.1047.
The integrin αIIbβ3 on circulating platelets is constrained in an inactive conformation by intramolecular interactions involving the stalk, transmembrane, and cytoplasmic domains of its αIIb and β3 subunits to prevent spontaneous platelet aggregation in the circulation.2 However, at sites of vascular trauma, these interactions are disrupted nearly instantaneously, shifting αIIbβ3 to its active ligand binding conformation and enabling it to mediate the formation of hemostatic plugs. In an elegant series of experiments, the Shattil and Ginsberg laboratories have shown that binding of the FERM (4.1/ezrin/radixin/moesin) domain of the cytoskeletal protein talin-1 to the β3 cytoplasmic tail is the event that disrupts the αIIbβ3 constraints.3 Further, they have proposed that a complex containing the active form of the low-molecular-weight GTP-binding protein Rap-1 and RIAM (Rap1-GTP interacting adapter molecule) delivers talin-1 to β3.4 Thus the complex connects platelet agonist–stimulated Rap-1 activation to αIIbβ3 activation. Importantly, the complex also appears to expose the β3-binding site on the talin-1 FERM domain, which is otherwise masked in the absence of talin-1 proteolysis or phosphorylation.
These experiments have suggested that talin-1 binding to β3 alone is sufficient, at least in vitro, to cause αIIbβ3 activation.5 Thus the observation that mutations in the gene for kindlin-3 in patients with LAD-III (leukocyte adhesion deficiency-III) produce a platelet phenotype resembling Glanzmann thrombasthenia was puzzling.6,7 Talin-1 and kindlin-3 both bind to the β3 cytoplasmic tail, but at separate sites, and in contrast to talin-1, kindlin-3 binding alone does not cause αIIbβ3 activation. Thus it is conceivable that kindlin-3 binding “primes” the β3 cytoplasmic tail for talin-1 binding, although this has yet to be shown convincingly. Alternatively, the Ginsberg/Shattil laboratories have proposed that kindlins may act to increase the affinity of integrins for multivalent ligands like fibrinogen by promoting the clustering of talin-activated integrins.8
In an earlier publication in Blood, Kasirer-Friede et al reported that agonist-stimulated αIIbβ3 activation was decreased in mouse platelets lacking the adapter protein ADAP.9 This implied that ADAP is a component of the signal transduction pathways that activate αIIbβ3. ADAP is a 120 or 130 kDa alternatively-spliced adapter protein expressed in hematopoietic cells whose function has been studied extensively in T lymphocytes. In T cells, ADAP is present in a membrane-associated complex containing SKAP1 (Src kinase-associated phosphoprotein 1, SKAP-55) and RIAM.10 After T-cell receptor activation, activated Rap1 binds to RIAM in the complex and in turn causes activation of β2- and β1-containing integrins. The role of ADAP in the complex appears to be to target SKAP1-RIAM to the membrane and to stabilize the otherwise unstable SKAP1 protein.
Platelets do not contain a complex analogous to ADAP-SKAP1-RIAM. However, because ADAP does promote agonist-stimulated αIIbβ3 activation, Kasirer-Friede et al hypothesized that ADAP acts by binding to either talin-1 or kindlin-3, or to both. In this paper, they show that indeed ADAP binds to talin-1 and to kindlins via interactions that do not involve either the SKAP1-homolog SKAP2 present in platelets or RIAM. Talin and kindlin binding to ADAP was detected in unstimulated platelets but decreased after platelet stimulation, suggesting that platelet stimulation induced the transfer of talin and kindlin from ADAP to the β3 tail. By microscopy, they found that ADAP co-localized with talin and kindlin-3 in platelets spread on fibrinogen and with αIIbβ3, talin, and kindlin in SFLLRN-stimulated Chinese hamster ovary (CHO) cells. A puzzling feature of kindlin biology has been that although kindlin-2 overexpression in CHO cells supports αIIbβ3 activation, kindlin-3 overexpression does not. However, the authors found that co-expressing ADAP with kindlin-3 now allowed kindlin-3 to activate αIIbβ3, suggesting that the culprit was the absence of ADAP. Finally, they compared αIIbβ3 function in ADAP+/+ and ADAP−/− mice and found that ADAP deletion impaired agonist-stimulated fibrinogen binding, reduced agonist-stimulated αIIbβ3 “avidity modulation,” and reduced the fraction of αIIbβ3-bound fibrinogen that becomes irreversibly bound over time.
The novel results reported by Kasirer-Friede et al imply that ADAP acts as a scaffold, bringing talin and kindlin to the platelet membrane and presenting them to β3 to promote αIIbβ3 activation (see figure). Thus they have supplied yet another piece to the puzzle of αIIbβ3 activation and a new direction for future research. In addition, their results raise a number of interesting questions. What is the relative contribution of the ADAP- and RIAM-associated pathways to αIIbβ3 activation? How do agonists stimulate the ADAP-associated pathway? How does ADAP associate with the platelet membrane, and are intermediary proteins involved? Finally, do talin and kindlin bind directly to ADAP or are as yet unidentified proteins analogous to SKAP1 and RIAM required? This platelet biologist eagerly looks forward to the answers to these questions.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal