Key Points
The immune receptor FcγRIIa is a key mediator of tumor cell activation of platelets in the circulation.
Secretion of adenosine 5′-diphosphate from dense granules is the primary response of platelets to activation by tumor cells.
Abstract
Platelets play a role in cancer by acting as a dynamic reservoir of effectors that facilitate tumor vascularization, growth, and metastasis. However, little information is available about the mechanism of tumor cell–induced platelet secretion (TCIPS) or the molecular machinery by which effector molecules are released from platelets. Here we demonstrate that tumor cells directly induce platelet secretion. Preincubation of platelets with human colon cancer (Caco-2), prostate cancer (PC3M-luc), or breast cancer cells (MDA-MB-231;MCF-7) resulted in a marked dose-dependent secretion of dense granules. Importantly, TCIPS preceded aggregation which always displayed a characteristic lag time. We investigated the role of platelet receptors and downstream molecules in TCIPS. The most potent modulators of TCIPS were the pharmacologic antagonists of Syk kinase, phospholipase C and protein kinase C, all downstream mediators of the immunoreceptor tyrosine-based activation motif (ITAM) cascade in platelets. Supporting this, we demonstrated a central role for the immune Fcγ receptor IIa (FcγRIIa) in mediating platelet-tumor cell cross-talk. In conclusion, we demonstrate that cancer cells can promote platelet dense-granule secretion, which is required to augment platelet aggregation. In addition, we show a novel essential role for FcγRIIa in prostate cancer cell–induced platelet activation opening the opportunity to develop novel antimetastatic therapies.
Introduction
There is increasing evidence supporting an association between cancer growth, metastasis, and platelet secretion.1-4 An association between thrombocytosis and cancer death was first reported in 1872 by Reiss and colleagues5 and brought to our attention again in 1964.6 Boneu et al, in 1984,7 first reported the presence of degranulated platelets, characterized by low levels of adenosine 5′-diphosphate (ADP) and serotonin, in patients with malignant solid tumors, while others reported an inverse relationship between cancer prognosis and platelet count in patients.8 Later, Karpatkin and Pearlstein demonstrated that many tumor cells could directly activate platelets.9
Platelet depletion in tumor-bearing mice triggers intratumor hemorrhage which is rescued by platelet secretome,1 demonstrating a requirement for platelet secretion to stabilize angiogenesis in tumor metastases. Indeed, platelet α-granules contain large reservoirs of cytokines and growth factors, including transforming growth factor β (TGF-β) and vascular endothelial growth factor (VEGF),10,11 which are selectively released upon platelet activation.12,13 Consistent with a role of platelet secretion in tumor metastasis, TGF-β derived from platelet α-granules has been shown to profoundly impact tumor metastasis and survival by enhancing an epithelial mesenchymal-like transition and suppressing tumor cell natural killer–mediated lysis, respectively.2,3 Moreover, platelet secretion in a tumorigenic microenvironment seems to differ materially from wound-induced platelet secretion. Battinelli and colleagues demonstrated the capability of cancer cells to provoke the preferential release of proangiogenic mediators from platelet α-granules,4 creating a dynamic microenvironment favorable for their growth and survival. Although considerable progress has been made in understanding the role that platelet secretion plays in tumor metastasis, very little is known about the ability of cancer cells to induce platelet granule release. Moreover, the precise molecular mediators of this phenomenon remain unknown.
In the present study, we had 2 specific objectives. First, we aimed to investigate the ability of cancer cells to elicit platelet secretion using a pair of human prostate cancer cell lines: PC3 and its highly metastatic variant PC3M; a pair of breast cancer cell lines, MCF-7 (low metastatic) and MDA-MB-231 (high metastatic); and colorectal cancer cells Caco-2, whose ability to induce platelet activation in vitro has been well characterized.14,15 Second, we sought to elucidate the molecular pathway(s) that mediate platelet activation in response to metastatic prostate cancer cells, PC3M, using a pharmacologic approach. In a context of molecular divergence, we propose a novel role for platelet Fcγ receptor IIa (FcγRIIa) and downstream molecules as key elements in platelet activation induced by metastatic prostate cancer cells.
Material and methods
Reagents
All reagents were purchased from Sigma-Aldrich unless otherwise indicated. Thrombin receptor–activating peptide (TRAP) was obtained from Bachem. CHRONO-LUME reagent and the ristocetin cofactor assay were purchased from Chrono-log (Labmedics Limited). SQ29548 was obtained from Enzo Life Sciences. Aspirin, GF-109203X, AACOCF3, LY294, PF228, PP2, piceatannol, U73122, and wortmannin were purchased from Tocris Bioscience. Abciximab (ReoPro) was provided from Centocor/Lilly. The monoclonal antibody (mAb) IV.3 Fab fragment against the FcγRIIa was kindly provided by Prof P. Newman (BloodCenter Wisconsin).
Tumor cell culture
Caco-2 cells, a human epithelial colorectal adenocarcinoma cell line, PC3M-luc and PC3, and metastatic and nonmetastatic human prostate cancer cells were a generous gift from Prof Marek W. Radomsky (Trinity College Dublin [TCD], Dublin, Ireland) and Prof Judy Harmey (Royal College of Surgeons in Ireland [RCSI], Dublin, Ireland), respectively. MCF-7 and MDA-MB-231 breast cancer cells were kindly provided by Dr Ann Hopkins (Beaumont Hospital, Dublin, Ireland). Caco-2 cells were grown as previously described.15 PC3M-luc and MCF-7 were grown in modified Eagle medium (MEM) supplemented with 10% of fetal bovine serum (FBS). PC3 were grown in RPMI 1640 with 10% FBS. MDA-MB-231 were grown in Dulbecco MEM with 10% FBS. When confluent, cells were detached using Accutase (Sigma-Aldrich).
Preparation of human and mouse washed platelets
Human blood was drawn on the day of the experiment from healthy, drug-free volunteers into 1:9 (v/v) sodium citrate anticoagulant (3.15%) with informed consent, in accordance with the Declaration of Helsinki. Ethical approval was obtained from the RCSI ethics committee. Washed platelet (WP) suspension (300 × 103 platelets per μL) was prepared as previously described.16 Mouse WPs were drawn and prepared as previously described.17,18
Platelet aggregation assay
Platelet aggregation was monitored at 37°C with continuous stirring in a PAP-4/8 aggregometer (Bio-Data Corporation). All aggregation experiments were measured against Tyrode buffer as the blank. Platelets (300 × 103 platelets per μL) were aggregated in the presence of cancer cells for 30 minutes or twice the lag time taken for the onset of aggregation response, whichever was longest. Where inhibitors were used, platelets were preincubated for 15 minutes at 37°C, 300 rpm, prior to commencement of aggregation. The data are expressed either as the percentage of aggregation or as the area under the aggregation curve (AUC). Data are normalized to the maximal aggregation achieved with each agonist. The effect of the treatment on platelet aggregation was expressed as the percentage of the control sample activated with agonist or tumor cells alone. Control samples were incubated with the corresponding volume of buffer or with either 0.1% dimethylsulfoxide (MRS2395, MRS2179, wortmannin, LY294, PP2, PF228, Bay-61, piceatannol, U73122, GF-109203X) or ethanol (SQ29548 and AACOCF3) used as vehicle for the reported inhibitors.
Platelet-dense granule secretion assay
Platelet ADP/adenosine triphosphate (ATP) secretion (PAS) from dense granules was assessed as previously described19,20 and monitored in parallel with platelet aggregation on the same donors. Briefly, increasing (0.1-100 × 103 cells per mL) or maximum (100 × 103 cells per mL) concentrations of cancer cells were dispensed in a final volume of 10 µL into different wells of a 96-well white plate (Sigma-Aldrich) and 80 μL of WP were gently pipetted on top. TRAP (10 μM) was used as positive control. Cancer cells were then allowed to activate platelets for a maximum of 30 minutes at 37°C under fast orbital shake. Finally, 10 μL of the detection reagent Chronolume were added to the wells and the sample luminescence was detected using a Wallac 1420 multilabel counter (Perkin Elmer). Where inhibitors were used, WPs were pretreated as in the “Platelet aggregation assay” section. Data are expressed as a percentage of a maximal response (% MaxPAS; arbitrary unit [AU]) as for aggregation.
P-selectin exposure
α-granule release was measured by P-selectin exposure. Washed human platelets at 300 × 103 platelets per μL were preincubated at 37°C, 300 rpm, for 10 minutes with 50 μg/mL of the αIIbβ3 inhibitor abciximab (to prevent platelet aggregation) prior to conducting the test response. Platelets were subsequently stimulated with either TRAP or tumor cells (100 × 103 cells per mL) as outlined in the previous section. Following incubation, platelets were stained with phycoerythrin mouse anti-human CD62P and analyzed by flow cytometry (Becton Dickinson).
Cancer cell adhesion to platelets
WPs (100 × 103 platelets per μL) were coincubated with cancer cell suspensions (106 cells per mL) at 37°C for 10 to 20 minutes according to the cell line used. Adhered platelets were labeled with a fluoresceinated (fluorescein isothiocyanate) antibody directed against glycoprotein IIb (GpIIb) (CD41a) for 15 minutes at room temperature. Fluorescence was determined as outlined in the previous paragraph.
Phosphorylation of integrin β3
The phosphorylation of β3 integrin was determined from a method adapted from Law et al.21 Briefly, washed platelets were stimulated as in the “Platelet aggregation assay” section, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotted for phosphointegrin β3 (pY759.7A; Santa Cruz Biotechnology). After stripping, equal loading was verified by immunoblotting with integrin β3 antibody (Cell Signaling).
Statistical analysis
Data were analyzed using GraphPad PRISM 4.0 software. Results are expressed as the mean ± standard error (SEM). Analysis of means was performed with a 2-tailed Student t test. Differences were considered significant at P < .05.
Results
Tumor cell–induced physiological platelet aggregation
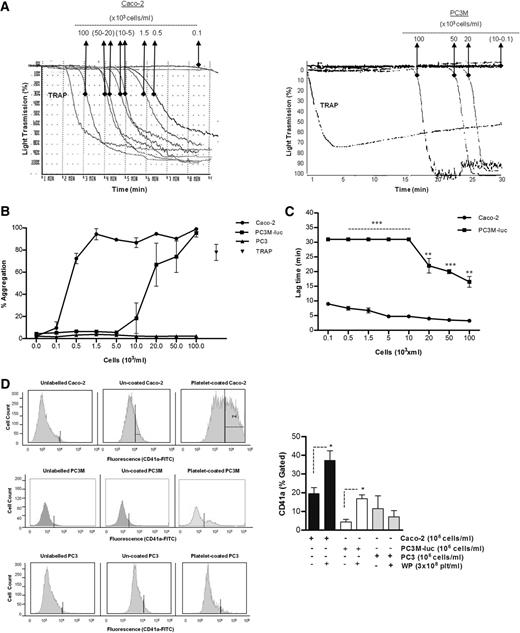
The ability of cancer cells to induce platelet aggregation was analyzed using Caco-2 human colorectal cancer cells, and 2 variants of a human prostate carcinoma: the metastatic PC3M-luc cells and their nonmetastatic variant subline,PC3.22 Representative results for the aggregation of washed human platelets by cancer cells are shown in Figure 1A-B. A dose-dependent platelet aggregation occurred in presence of both Caco-2 and PC3M-luc cells. Prostate cancer cells (PC3M-luc) failed to induce aggregation at lower concentration (0.1-10 × 103 cells per mL) while colon cancer cells were able to stimulate powerful aggregation at all the concentrations tested. However, the maximum aggregation was identical at the highest cell concentration used (100 × 103cells per mL). In contrast, the nonmetastatic PC3 cells were incapable of triggering platelet aggregation (Figure 1B).
Tumor cell–induced platelet aggregation. (A) Representative traces showing a dose-dependent platelet aggregation response to increasing concentrations (as indicated) of Caco-2 (left trace) or PC3M-luc cells (right trace). TRAP (10 μM) was used as control of the platelet response. These tracings represent fragments of the aggregometry output focusing on the time where most changes in platelet responses are observed. (B) Corresponding dose-response curve displaying the concentration response of WP (300 × 103 platelets per μL) to increasing concentration of Caco-2 (n = 6), PC3M-luc (n = 6), or PC3 (n = 3) cells. TRAP (10 μM) was used as positive control (data are expressed as mean % Agg ± SEM). (C) Representative curves comparing the lag time in aggregation of Caco-2 and PC3M-luc (n = 4). PC3M-luc–triggered WP aggregation was monitored for 30 minutes per experiment. If aggregation failed to occur during this time period, a lag time of 31 minutes was recorded (data are expressed as mean ± SEM; **P < .01; ***P < .0001). (D) The direct interaction of washed platelets with cancer cells was measured by flow cytometry based on CD41a (platelet-specific surface marker) expression on cancer cells. Cells were gated by forward/side scatter characteristic to exclude cellular debris and platelets. The percentage of CD41a binding was determined in the cell gate as percentage gated, and the fold increase in cancer cells preincubated with platelets compared with cancer cells alone was recorded. Flow cytometry histograms are representative of 4 (PC3M/Caco-2) or 3 (PC3) independent experiments that yielded similar results (*P < .05, n = 4/3, mean ± SEM).
Tumor cell–induced platelet aggregation. (A) Representative traces showing a dose-dependent platelet aggregation response to increasing concentrations (as indicated) of Caco-2 (left trace) or PC3M-luc cells (right trace). TRAP (10 μM) was used as control of the platelet response. These tracings represent fragments of the aggregometry output focusing on the time where most changes in platelet responses are observed. (B) Corresponding dose-response curve displaying the concentration response of WP (300 × 103 platelets per μL) to increasing concentration of Caco-2 (n = 6), PC3M-luc (n = 6), or PC3 (n = 3) cells. TRAP (10 μM) was used as positive control (data are expressed as mean % Agg ± SEM). (C) Representative curves comparing the lag time in aggregation of Caco-2 and PC3M-luc (n = 4). PC3M-luc–triggered WP aggregation was monitored for 30 minutes per experiment. If aggregation failed to occur during this time period, a lag time of 31 minutes was recorded (data are expressed as mean ± SEM; **P < .01; ***P < .0001). (D) The direct interaction of washed platelets with cancer cells was measured by flow cytometry based on CD41a (platelet-specific surface marker) expression on cancer cells. Cells were gated by forward/side scatter characteristic to exclude cellular debris and platelets. The percentage of CD41a binding was determined in the cell gate as percentage gated, and the fold increase in cancer cells preincubated with platelets compared with cancer cells alone was recorded. Flow cytometry histograms are representative of 4 (PC3M/Caco-2) or 3 (PC3) independent experiments that yielded similar results (*P < .05, n = 4/3, mean ± SEM).
Interestingly, a sustained lag time was observed prior to the onset of a brisk aggregation in the presence of both metastatic cancer cell lines. This lag time decreased as the concentration of cancer cells increased (Figure 1C). At the highest concentration of cancer cells, the lag time measured with PC3M-luc–stimulated platelets was significantly longer compared with that recorded with Caco-2 cells (mean ± SEM; 16.5 ± 1.8 minutes vs 3.3 ± 0.3 minutes; **P < .01, n = 6; 100 × 103 cells per mL).
To address whether platelets could bind directly to the cancer cells, we quantitatively evaluated the association of the platelet-specific protein, CD41a, with the cancer cells following coincubation. Flow cytometric analysis showed that PC3M, PC3, and Caco-2 cells are negative for CD41a expression. However, CD41a expression is associated with Caco-2 and PC3M cells, but not PC3, following preincubation with platelets (Figure 1D), providing compelling evidence that platelet aggregation–inducing cancer cells are physically interacting with platelets.
To verify that a physiological aggregation was occurring, the ristocetin cofactor assay was performed and PC3M-luc cells were assessed for their ability to agglutinate lyophilized platelets in the presence of von Willebrand factor (VWF)–rich plasma. Importantly, no agglutination could be observed when lyophilized platelets were stimulated with prostate cancer cells (100 × 103 cells per mL) or TRAP (10 μM) over a period of 30 minutes in the presence of VWF (supplemental Figure 1A, available on the Blood Web site). Altogether, these results confirmed that physiological aggregation, and not passive agglutination was being observed in response to cancer cells.
Tumor cell–induced physiological platelet secretion
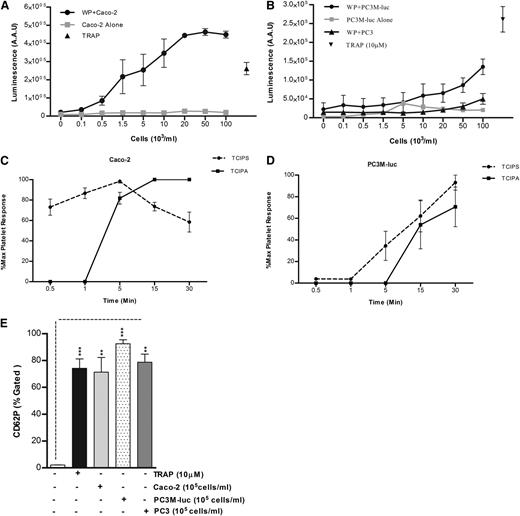
Because it has been previously shown that platelets are an important source of bioavailable VEGF4 and TGFβ3 and because evidence has been provided that platelet secretion is crucial for platelet regulation of tumor homeostasis,1 the capability of tumor cells to induce platelet secretion in vitro was explored using a luminometric assay normally used to detect the platelet ATP/ADP secretion (PAS) by dense granules.19 TRAP (10 μM) was used as positive control. No ATP/ADP was detected following incubation of cancer cells with Tyrode buffer in the absence of platelets. As evident from Figure 2, cancer cells caused a dose-dependent release of significant amounts of ATP/ADP from platelet-dense granules, with a greater release in presence of Caco-2 (Figure 2A) than PC3M-luc cells (Figure 2B), confirming the results obtained in aggregometry (Figure 1B). The nonmetastatic PC3 cell variant failed to induce platelet-dense granule secretion. Similar results reflecting an association between the metastatic potential and the ability to elicit platelet secretion and aggregation were observed using a pair of breast cancer cells (MCF-7 and MDA-MB-231) that differ in their metastatic ability22 (supplemental Figure 2). The lag time for tumor cell–induced platelet secretion was assessed in response to the highest dose of cancer cells (100 × 103 cells per mL) for several time points (30 seconds–30 minutes). Caco-2 cells promoted an almost instantaneous platelet secretion (Figure 2C). In contrast, the response to PC3M-luc cells was slower and was not apparent until 5 minutes after the coincubation with platelets (Figure 2D). We suggest therefore that tumor cell–induced platelet secretion (TCIPS) occurred before tumor cell–induced platelet aggregation (TCIPA).
TCIPS preceded aggregation. (A-B) WPs (300 × 103 platelets per μL) were stimulated with increasing concentration (0.1-100 × 103 cells per mL) of Caco-2 (A, n = 6), PC3M-luc (B, n = 6), or PC3 cells (B, n = 3) and representative dose-response profile for luminescence arbitrary absorbance unit (AAU) deriving from platelet ATP/ADP release are shown. The lines in gray display the amount of ATP/ADP released by cancer cells in the absence of any platelets. TRAP (10 μM)–stimulated platelets were used as positive control (data are expressed as mean ± SEM). (C-D) Progress time curves of TCIPS and aggregation (TCIPA; n = 6). The curves precisely depict the temporal relationship between platelet secretion and aggregation induced by max concentration (100 × 103 cells per mL) of Caco-2 (C) or PC3M-luc (D) cells. Values (mean ± SEM) are expressed as the percentage of the maximum platelet response, identifiable with the percentage of the highest platelet secretion or aggregation measured for each donor at the indicated time point. (E) Platelet α-granule release in response to maximum concentration of cancer cells (100 × 103 cells per mL) was measured by quantifying changes in CD62P surface expression in flow cytometry (***P < .0001; **P < .01; mean ± SEM). Each experiment was repeated 4 times in presence of PC3M and Caco-2 and 3 times in presence of PC3 cells. The level of platelets expressing P-selectin was defined as a fraction of the 10 000 platelets that exhibit specific CD62P binding. The percentage of CD62P binding was determined as the percentage gated and the fold increase in cancer cell–stimulated platelets compared with resting platelets was recorded. AAU, arbitrary absorbance unit.
TCIPS preceded aggregation. (A-B) WPs (300 × 103 platelets per μL) were stimulated with increasing concentration (0.1-100 × 103 cells per mL) of Caco-2 (A, n = 6), PC3M-luc (B, n = 6), or PC3 cells (B, n = 3) and representative dose-response profile for luminescence arbitrary absorbance unit (AAU) deriving from platelet ATP/ADP release are shown. The lines in gray display the amount of ATP/ADP released by cancer cells in the absence of any platelets. TRAP (10 μM)–stimulated platelets were used as positive control (data are expressed as mean ± SEM). (C-D) Progress time curves of TCIPS and aggregation (TCIPA; n = 6). The curves precisely depict the temporal relationship between platelet secretion and aggregation induced by max concentration (100 × 103 cells per mL) of Caco-2 (C) or PC3M-luc (D) cells. Values (mean ± SEM) are expressed as the percentage of the maximum platelet response, identifiable with the percentage of the highest platelet secretion or aggregation measured for each donor at the indicated time point. (E) Platelet α-granule release in response to maximum concentration of cancer cells (100 × 103 cells per mL) was measured by quantifying changes in CD62P surface expression in flow cytometry (***P < .0001; **P < .01; mean ± SEM). Each experiment was repeated 4 times in presence of PC3M and Caco-2 and 3 times in presence of PC3 cells. The level of platelets expressing P-selectin was defined as a fraction of the 10 000 platelets that exhibit specific CD62P binding. The percentage of CD62P binding was determined as the percentage gated and the fold increase in cancer cell–stimulated platelets compared with resting platelets was recorded. AAU, arbitrary absorbance unit.
α-granules release was next assessed. Platelet stimulation with maximum concentration (100 × 103cells per mL) of cancer cells resulted in a marked increase in P-selectin surface expression within 10 to 15 minutes after stimulation (Figure 2E). Interestingly, while PC3 cells failed in inducing platelet aggregation and dense-granule secretion, a significant P-selectin exposure was observed. This interesting result suggests that cancer cell–platelet cross-talk can occur also in absence of a metastatic phenotype.
Finally, to ascertain that a physiological secretion was occurring, the amount of cytoplasmic lactate dehydrogenase released by PC3M-luc–stimulated platelets was measured. As evident from supplemental Figure 1B, no lactate dehydrogenase was detected after platelet activation with either TRAP (10 μM) or maximal concentration of PC3M-luc cells, strongly confirming that physiological secretion, not passive lysis, was being observed.
PC3M-induced platelet response is PAR-1, GpIb, and thromboxane receptor independent
As shown in Figure 2, cancer cell–induced platelet secretion is a primary event followed by brisk aggregation. However, the knowledge of the precise molecular mediators responsible for the platelet-tumor interaction remains unclear. In the present study, we specifically explored the platelet pathways required by the PC3M cells that give rise to secretion and aggregation by targeting potential platelet receptors and downstream mediators with pharmacologic inhibitors. In general, the concentration of pharmacologic inhibitors and blocking antibodies was chosen with regard to previous platelet studies.23-25 In addition, dose-response curves were generated for selective antagonists (supplemental Figure 3).
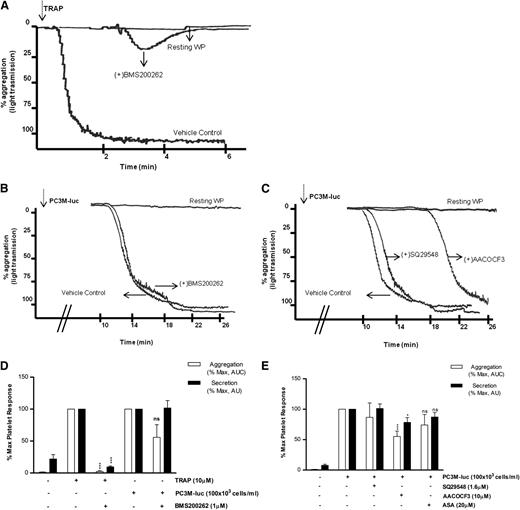
The first question to be addressed was the nature of the receptor required by the cancer cells to activate human platelets. In contrast to TRAP-activated platelets (Figure 3A,D), blockade of PAR-1 with BMS200261 had no significant effect on the extent of maximal aggregation and secretion induced by PC3M-luc cells (Figure 3B-D). These data suggest that PAR-1 activation itself is not a component of prostate cancer–induced platelet activation. We next investigated whether GpIb, the well-characterized thrombin and VWF receptor, played a role in TCIPS. For this purpose, we used a range of antibodies targeting different motifs within the GpIb molecule.24 Importantly, GpIb inhibition did not affect PC3M-luc–induced platelet secretion (supplemental Figure 4).
PAR-1 and thromboxane receptors do not mediate TCIPA or TCIPS. (A-C) Representative aggregation traces showing the effect of the reported inhibitors on TRAP (10 μM; A)– or PC3M-luc (100 × 103 cells per mL; B-C)–induced platelet aggregation. (D-E) Bar graph comparing the effect of the indicated inhibitors on platelet aggregation (n = 3/9) and secretion (n = 6/10) elicited by TRAP or PC3M-luc. Results were normalized for each donor relative to the maximal response seen in TRAP- or PC3M-luc–stimulated platelets (100%) in presence of the vehicle solution (data are expressed as mean ± SEM; *P < .05; ***P < .0001).
PAR-1 and thromboxane receptors do not mediate TCIPA or TCIPS. (A-C) Representative aggregation traces showing the effect of the reported inhibitors on TRAP (10 μM; A)– or PC3M-luc (100 × 103 cells per mL; B-C)–induced platelet aggregation. (D-E) Bar graph comparing the effect of the indicated inhibitors on platelet aggregation (n = 3/9) and secretion (n = 6/10) elicited by TRAP or PC3M-luc. Results were normalized for each donor relative to the maximal response seen in TRAP- or PC3M-luc–stimulated platelets (100%) in presence of the vehicle solution (data are expressed as mean ± SEM; *P < .05; ***P < .0001).
Previously, a role for Thromboxane A2 (TxA2) receptor inhibitors in selectively ablating human osteogenic sarcoma (MG63)–induced platelet aggregation in vitro was suggested.26,27 In contrast, recent studies demonstrated the ineffectiveness of acetyl salicylic acid (ASA), a well-known inhibitor of TxA2 synthesis inhibitor, in reducing TCIPA in the presence of either breast cancer (MCF-7) or colon cancer cells (Caco-2).15,28 We therefore evaluated the effects of modulators of TxA2 on PC3M TCIPA and TCIPS. As evident in Figure 3C,E, preincubation of human WP with SQ29548 (1.6 μM), a selective inhibitor of TxA2 receptors, did not inhibit either tumor cell–induced platelet aggregation or secretion. Pretreatment of platelets with AACOCF3(10 μM), a cPLA2 inhibitor, resulted in a small but significant inhibition in the secretion (% MaxPAS; mean ± SEM: 100 vs 78.41 ± 8.7; P < .05, n = 10; Figure 3E) and aggregation response (% MaxAUC; mean ± SEM: 100 vs 55.2 ± 8.9; ***P < .001, n = 9; Figure 3C,E) associated with a delay in the onset of TCIPA when compared with the vehicle control. Finally, inhibition of TxA2 generation by platelets by ASA (20 μM) failed to induce a significant decrease in PC3M-luc–induced platelet secretion and aggregation (Figure 3E). Overall, this suggests that the thromboxane synthesis pathway is not a critical player in PC3M-cell activation of platelets. For this set of inhibitors, and all subsequent inhibitors, the effects on TRAP-induced platelet secretion and aggregation are shown in supplemental Figure 5.
P2Y12 and P2Y1 receptors do not contribute to prostate cancer cell–induced platelet secretion
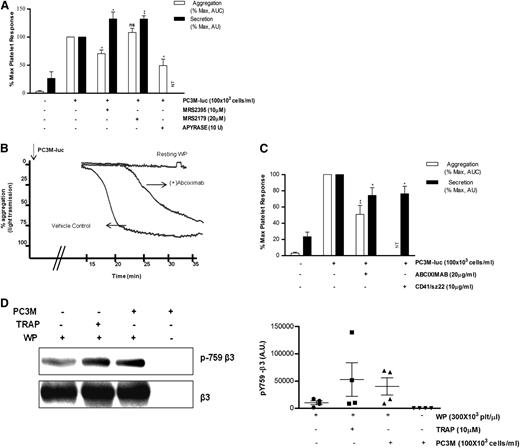
Because platelet secretion preceded aggregation, we were interested to know whether inhibitors of ADP receptors might have a differential effect on TCIPA/S. In agreement with the literature,28,29 the P2Y12 inhibitor (MRS2395,10 μM) or the ATP/ADP scavenger (Apyrase, 10 U/mL), but not the P2Y1 inhibitor (MRS2179,20 μM), caused consistent changes in the slope of the aggregation curve and significantly inhibited (% MaxAUC: MRS2395, 100 vs 70.5 ± 6.5; Apyrase, 100 vs 48.2 ± 11.5; *P < .05, n = 3; Figure 4A) the PC3M-luc–induced platelet aggregation response. Thus, TCIPA was sensitive to the ADP-P2Y12–deriving signaling pathway. Surprisingly, platelets preexposed to MRS3295 or MRS2179, before stimulation with PC3M-luc cells, showed a significant enhancement (% MaxPAS: 100 vs 132.3 ± 12.3; 100 vs 132.1 ± 5.6; **P < .01, n = 5; Figure 4A) of TCIPS. These findings suggest that ADP, secreted by platelets in response to PC3M-luc cells, triggers a positive feedback loop of activation and dense-granule secretion, culminating in full platelet aggregation.
ADP receptor blockers attenuate TCIPA but potentiate TCIPS, whereas inhibition of αIIbβ3 affects both. (A,C) Bar graph comparing the effect of the indicated inhibitors on platelet aggregation (n = 3/6; **P < .01) and dense-granule secretion (n = 5/6; *P < .05) elicited by PC3M-luc. CD41/SZ22 antibody (Beckman Coulter, UK), was tested in platelet secretion but not aggregation. Results are expressed and normalized for each donor as explained in the Figure 3 legend (data are expressed as mean ± SEM). (B) Representative aggregation trace showing the effect of the reported inhibitors on TCIPA elicited by PC3M-luc (100 × 103 cells per mL). (D) β3 integrin is phosphorylated following platelet stimulation with PC3M. Lysates from resting platelets or platelets (300 × 103 platelets per μL) activated with either TRAP (10μM) or PC3M cells (100 × 103 cells per mL) were immune-blotted with a phospho-specific integrin β3 antibody (pY759) as indicated. Loading protein levels were determined after stripping and reprobing of the same gels using a pan-β3 antibody. (Left panel) The band intensities are estimated by densitometry, (right panel) adjusted for differences in loading levels as detected by pan β3. Data are expressed as AU and represent mean ± SEM for 4 independent experiments. AU, arbitrary unit; NT, not tested.
ADP receptor blockers attenuate TCIPA but potentiate TCIPS, whereas inhibition of αIIbβ3 affects both. (A,C) Bar graph comparing the effect of the indicated inhibitors on platelet aggregation (n = 3/6; **P < .01) and dense-granule secretion (n = 5/6; *P < .05) elicited by PC3M-luc. CD41/SZ22 antibody (Beckman Coulter, UK), was tested in platelet secretion but not aggregation. Results are expressed and normalized for each donor as explained in the Figure 3 legend (data are expressed as mean ± SEM). (B) Representative aggregation trace showing the effect of the reported inhibitors on TCIPA elicited by PC3M-luc (100 × 103 cells per mL). (D) β3 integrin is phosphorylated following platelet stimulation with PC3M. Lysates from resting platelets or platelets (300 × 103 platelets per μL) activated with either TRAP (10μM) or PC3M cells (100 × 103 cells per mL) were immune-blotted with a phospho-specific integrin β3 antibody (pY759) as indicated. Loading protein levels were determined after stripping and reprobing of the same gels using a pan-β3 antibody. (Left panel) The band intensities are estimated by densitometry, (right panel) adjusted for differences in loading levels as detected by pan β3. Data are expressed as AU and represent mean ± SEM for 4 independent experiments. AU, arbitrary unit; NT, not tested.
Integrin αIIbβ3 is required for PC3M-luc cell–induced platelet response
There is strong evidence supporting a role for platelet adhesion molecules, such as αIIbβ3, in tumor cell–induced platelet activation.30-32 Abciximab (20 μg/mL), a specific monoclonal blocking antibody targeting αIIbβ3, significantly inhibited platelet aggregation and secretion (% MaxAUC: 100 vs 51.0 ± 10.90; **P < .01, n = 6; % MaxPAS: 100 vs 74.2 ± 9.6; *P < .05, n = 6) upon exposure to metastatic prostate cancer cells (Figure 4B-C). Moreover, changes in the slope of the aggregation curve and a remarkable delay in the onset of aggregation in the treated sample were observed. Similar results were achieved with CD41/SZ22, an αIIbβ3 mAb, when tested in platelet-dense granule secretion. Further confirmation of αIIbβ3 activation was provided by western blotting analysis. We show that platelet integrin β3 is phosphorylated in response to PC3M (Figure 4D). This result reinforces the hypothesis that αIIbβ3 is a crucial molecular mediator of platelet activation upon cancer cell stimulus.33
Intracellular molecular mediators of TCIPA/S are divergently activated: role of PI3K, Src, and FAK
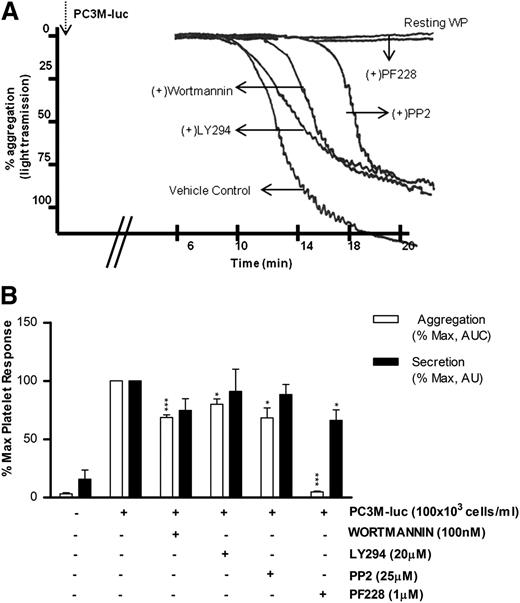
Phosphoinositide 3-kinase (PI3K) and Src kinase are downstream to a broad range of platelet and tumor cell pathways, whereas focal adhesion kinase (FAK) plays a key role in mediating downstream signals from integrins and growth factor receptors.34-37 However, the role of these intracellular platelet molecular mediators in the tumor cell–elicited signaling cascade remains unknown. As detailed in Figure 5, platelet aggregation induced by PC3M-luc was significantly (% MaxAUC: 100 vs 68.6 ± 2.3; ***P < .001, n = 5; 100 vs 80 ± 4.56; *P < .05, n = 4) affected by both wortmannin (100 nM) and LY294002 (20 μM), 2 unrelated PI3K inhibitors. In contrast, pharmacologic inhibition of PI3K did not impair TCIPS (Figure 5B).
PI3K and Src kinase are specifically activated during TCIPA, whereas FAK kinase function is required in TCIPS and TCIPA. (A) Representative aggregation trace showing the effect of inhibitors of Syk, PI3K, and FAK on PC3M-luc (100 × 103cells per mL) induced platelet aggregation. (B) Bar graph comparing the effect of the indicated inhibitors on platelet aggregation (n = 4/7; *P < .05; ***P < .0001) and dense-granule secretion (n = 4/7; *P < .05) elicited by PC3M-luc. Results are expressed and normalized for each donor as explained in the Figure 3 legend (data are expressed as mean ± SEM).
PI3K and Src kinase are specifically activated during TCIPA, whereas FAK kinase function is required in TCIPS and TCIPA. (A) Representative aggregation trace showing the effect of inhibitors of Syk, PI3K, and FAK on PC3M-luc (100 × 103cells per mL) induced platelet aggregation. (B) Bar graph comparing the effect of the indicated inhibitors on platelet aggregation (n = 4/7; *P < .05; ***P < .0001) and dense-granule secretion (n = 4/7; *P < .05) elicited by PC3M-luc. Results are expressed and normalized for each donor as explained in the Figure 3 legend (data are expressed as mean ± SEM).
The role of Src kinase in PC3M-luc activation of platelets is detailed in Figure 5. Whereas PP2 (25 μM), a broad-range inhibitor of Src-family kinases, did not alter the extent of TCIPS, it markedly reduced TCIPA (% MaxAUC: 100 vs 68.4 ± 8.6; *P < .05, n = 5). In contrast, FAK blockade, achieved using PF228, resulted in a dramatic reduction of TCIPA (% MaxAUC: 100 vs 7.81 ± 0.7; ***P < .0001, n = 4; Figure 5A-B) accentuated by a significant inhibition of TCIPS (% MaxPAS: 100 vs 66.1 ± 9.1; *P < .05, n = 7). Collectively, these results identify crucial intracellular molecular mediators of TCIPA/S, providing evidence for functional divergence in the signaling events involved in the activation of platelets upon exposure to metastatic prostate cancer cells. It is noteworthy that the profile of the pathways inhibited in our studies strongly overlap with pathways known to be involved in accessory signaling pathways that use immunoreceptor tyrosine-based activation motif (ITAM).
Dependence of cancer cell–induced platelet response on the FcγRIIa signaling pathway
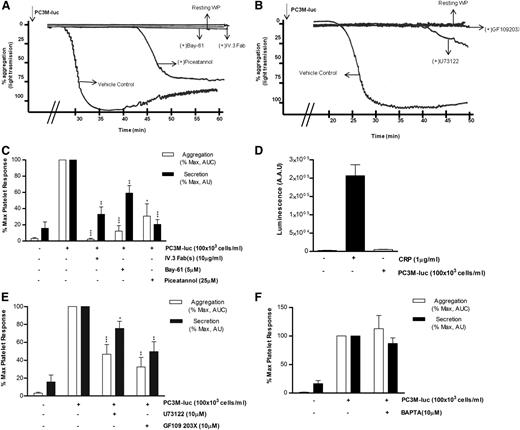
Considering the role that platelets play in the innate immune response38 and thinking of cancer cells as ultimate parasites of the bloodstream, we next investigated the possible involvement of the immune FcγRIIa, an ITAM-containing platelet receptor, in platelet activation induced by PC3M-luc. Washed platelets were preincubated with 10 μg/mL of the Fab fragments of the FcγRIIa blocking antibody IV.3 before being activated with PC3M-luc cells. The ability of tumor cells to induce aggregation (% MaxAUC: 100 vs 2.3 ± 1.2; ***P < .0001, n = 3) and dense granule secretion (% MaxPAS: 100 vs 32.9 ± 9.2; **P < .01) was markedly inhibited by antibody treatment (Figure 6A,C). Moreover, mouse platelets, which are naturally FcγRIIa negative, did not respond when stimulated with PC3M cells (Figure 6D). This result therefore identifies a new platelet receptor that is selectively required in PC3-M cancer cell–stimulated platelets by a mechanism, to date, completely unknown. Activation of human platelets by FcγRIIa is associated with rapid tyrosine phosphorylation and activation of the nonreceptor tyrosine kinase Syk.39 We compared the effect of Bay61-3606 (5 μM) and piceatannol (25 μM), 2 unrelated Syk inhibitors, on TCIPA and TCIPS and demonstrated dramatic inhibition of both responses (% MaxAUC; Bay-61: 100 vs 12.2 ± 6.8; ***P < .0001; piceatannol: 100 vs 30.7 ± 15.1; *P < .05; n = 6; % MaxPAS; Bay-61: 100 vs 59.1 ± 9.4; **P < .01; piceatannol: 100 vs 20.6 ± 5.8; ***P < .0001; n = 6; Figure 6) strongly supporting our proposal of a central role for FcγRIIa.
FcγRIIa, Syk, and PKC activation is necessary for PC3M to induce platelet aggregation and secretion. (A-B) Representative aggregation trace showing the effect of the reported inhibitors on PC3M-luc (100 × 103 cells per mL)–induced platelet aggregation. (C, E, F) Bar graph comparing the effect of the indicated inhibitors on platelet aggregation and dense-granule secretion (n = 4/9; *P < .05; **P < .01; ***P < .0001) elicited by PC3M-luc. Results are expressed and normalized for each donor as explained in the Figure 3 legend (data are expressed as mean ± SEM). (D) PC3M cells do not induce mouse platelet secretion. Mouse WPs (300 × 103 platelets per μL) were stimulated with PC3M cells (100 × 103 cells per mL) and results for luminescence, expressed as AAU, derived from platelet ATP/ADP release are shown. CRP (1 μg/mL)–stimulated platelets were used as the positive control (data are expressed as mean ± SEM).
FcγRIIa, Syk, and PKC activation is necessary for PC3M to induce platelet aggregation and secretion. (A-B) Representative aggregation trace showing the effect of the reported inhibitors on PC3M-luc (100 × 103 cells per mL)–induced platelet aggregation. (C, E, F) Bar graph comparing the effect of the indicated inhibitors on platelet aggregation and dense-granule secretion (n = 4/9; *P < .05; **P < .01; ***P < .0001) elicited by PC3M-luc. Results are expressed and normalized for each donor as explained in the Figure 3 legend (data are expressed as mean ± SEM). (D) PC3M cells do not induce mouse platelet secretion. Mouse WPs (300 × 103 platelets per μL) were stimulated with PC3M cells (100 × 103 cells per mL) and results for luminescence, expressed as AAU, derived from platelet ATP/ADP release are shown. CRP (1 μg/mL)–stimulated platelets were used as the positive control (data are expressed as mean ± SEM).
Phospholipase Cγ2 (PLCγ2) also lies downstream of the activated FcγRIIa-Syk pathway.40 The broad-spectrum PLC inhibitor, U73122(10μM) had a significant effect on tumor cell–induced platelet secretion (% MaxPAS: 100 vs 75.6 ± 7.9; *P < .05; n = 11) and effectively reduced and delayed TCIPA (% MaxAUC; 100 vs 40.7 ± 8.5; P < .0001; n = 9; Figure 6B,E). It is also noteworthy that the intracellular calcium antagonist 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA,10 μM) did not affect TCIPS or TCIPA response (n = 7/4; Figure 6F), suggesting a Ca2+-independent platelet activation. The inhibitory properties of BAPTA were verified in collagen-related peptide (CRP)–induced platelet aggregation (supplemental Figure 3F).
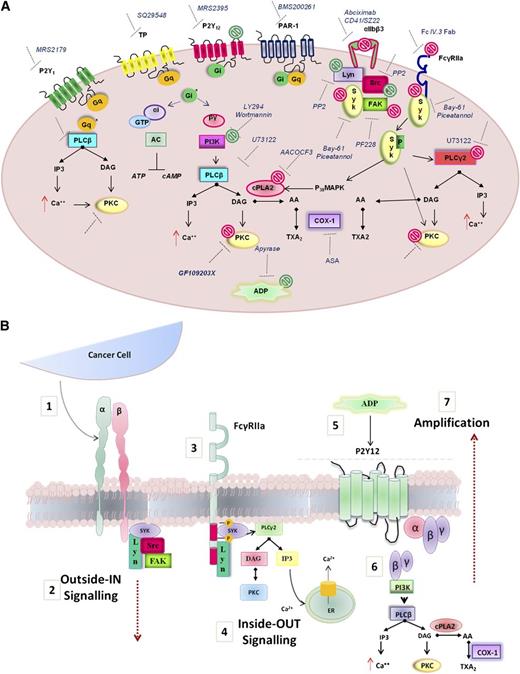
The protein kinase C (PKC) family includes many isoforms all differently involved in a number of platelet processes, most importantly aggregation and secretion.41 The possible role of PKC in PC3M-induced platelet aggregation and secretion was next explored. Human WPs were preincubated with GF109203X (10μM), a potent broad-spectrum PKC inhibitor and a marked decrease in both platelet aggregation and secretion responses to PC3M-luc cells were observed (Figure 6B,E; % MaxAUC; 100 vs 32.5 ± 10.7; **P < .01; n = 6; % MaxPAS; 100 vs 49, 68 ± 11, 02; **P < .01; n = 6), confirming the important functional profile of PKC in platelets. A general scheme of platelet-signaling pathways is summarized in Figure 7 showing all potential molecular mediators of TCIPA/S induced by metastatic PC3M prostate cancer cells and highlighting a central role for ITAM-dependent pathway(s).
Model depicting the molecular mediators of TCIPA/S induced by metastatic prostate cancer cells (PC3M). (A) The model shown depicts the activation pathway of key signaling molecules investigated in the present study. Pharmacologic inhibitors used to target receptors and signaling molecules in platelets are shown. The effects of these inhibitors are summarized by green symbols, which indicate inhibition of platelet aggregation only, or red symbols which indicate inhibition of both secretion and aggregation. (B) Hypothesized integrin αIIbβ3-FcγRIIa-P2Y12 cross-talk following platelet exposure to PC3M cells. (1) Upon αIIbβ3 engagement an (2) “outside-in ” signal transduction is transmitted possibly triggering the (3-4) activation of an “inside-out” FcγRIIa-deriving downstream signaling with consequent release (5) of secondary mediators, such as ADP. ADP engagement of its cognate receptor P2Y12 would result in the amplification of the response (6-7) converging in an augmented platelet aggregation.
Model depicting the molecular mediators of TCIPA/S induced by metastatic prostate cancer cells (PC3M). (A) The model shown depicts the activation pathway of key signaling molecules investigated in the present study. Pharmacologic inhibitors used to target receptors and signaling molecules in platelets are shown. The effects of these inhibitors are summarized by green symbols, which indicate inhibition of platelet aggregation only, or red symbols which indicate inhibition of both secretion and aggregation. (B) Hypothesized integrin αIIbβ3-FcγRIIa-P2Y12 cross-talk following platelet exposure to PC3M cells. (1) Upon αIIbβ3 engagement an (2) “outside-in ” signal transduction is transmitted possibly triggering the (3-4) activation of an “inside-out” FcγRIIa-deriving downstream signaling with consequent release (5) of secondary mediators, such as ADP. ADP engagement of its cognate receptor P2Y12 would result in the amplification of the response (6-7) converging in an augmented platelet aggregation.
Discussion
Once in the bloodstream, cancer cells perturb the surrounding microenvironment, triggering abnormal platelet responses5,6,42-44 mediated by direct3 or indirect interactions.4 Platelets can be aggregated in vitro and in vivo by various cancer cell lines,31 a phenomenon described in 1968 as “tumor cell induced platelet aggregation.”8 Many factors are secreted by platelet granules in response to tumor cells, such as TGF-β and VEGF, that support the metastatic cascade and promote tumor vascularization.3,4 Kerr and colleagues showed that many tumor-secreted proteins are taken up and stored in platelets and specifically released at the metastatic sites.13 However, while the ability of cancer cells to aggregate and cluster platelets around the tumor is supported by a large body of experimental and clinical data,32,45,46 the role of cancer cells in promoting platelet secretion of procarcinogenic and thrombotic factors is poorly understood. In the present study, we show that highly metastatic colorectal cancer cells (Caco-2) and a prostate carcinoma cell line (PC3M-luc) stimulate the release of platelet-dense granule contents in a dose-dependent manner. In contrast, cell lines with low metastatic potential including the isogenic PC3 prostate cancer cell line showed a markedly reduced response. A similar pattern of response was observed with a pair of breast cancer cell lines (supplemental Figure 2), emphasizing the hypothesis of a direct association between the metastatic potential and tumor cell–induced platelet aggregation and secretion.
A sustained α-granule release was also observed in response to all the cell lines tested. It is noteworthy that the nonmetastatic prostate cancer PC3 cells activated α-granule secretion with no effect on aggregation and dense granule release suggesting that nonmetastatic cancer cells can activate platelets without stimulating a prothrombotic response. A more precise interrogation of the comparative contents of the bioactive agents in the platelet α-granule releasate may allow a greater understanding of molecular events that ensue following activation of platelets with metastatic or nonmetastatic cells. Evaluation of platelet secretion over time showed that Caco-2 and PC3M-luc cancer cells induced platelet ATP/ADP release earlier than aggregation. The aggregation was characterized by a distinct lag time which correlated with the cell’s ability to induce TCIPS. Moreover, antagonism of platelet P2Y12 receptors with MRS2395 or scavenging of released ADP with apyrase significantly attenuated aggregation, but not secretion responses. Thus, these results strongly imply that cancer cell–induced release of dense granule ADP facilitates the induction of platelet aggregation and suggest that rapid dense-granule secretion is the primary platelet activation pathway in response to cancer cells. If this is indeed the case, then we can expect that all inhibitors of the secretory pathway(s) will also have a downstream impact on the platelet aggregation responses recorded. Thus, agents that inhibit platelet secretion of dense granules will de facto inhibit the dependent response, namely aggregation, which may result in reduced thrombotic complications in cancer patients.
We next explored the activity of a range of pharmacological inhibitors targeted at various platelet receptors and cytosolic signaling proteins. We were interested mainly in agents that inhibited both secretion, which we hypothesized to be a primary response, and aggregation, which would represent a secondary response. In this screen, inhibitors of integrin αIIbβ3, FAK, Syk kinase, and PKC showed significant effects on both TCIPS and TCIPA. These components have all previously been identified in association with each other in a platelet context.
FcγRIIa is an ITAM-containing receptor used in platelets for immune receptor signaling.47 The ITAM-signaling motif is also found in other receptors in platelets, such as the collagen receptor complex GPVI-FcR γ chain, and the podoplanin receptor CLEC-2. More recently, a role for the podoplanin-CLEC-2 axis in mediating tumor metastasis has been identified.48 FcγRIIa directly interacts with Syk, activating PKC either directly25 or via PLC activation.40 In our studies, FcγRIIa inhibition with the fab antibody IV.3 substantially blocked both platelet secretion and aggregation. Similarly, inhibition of the tyrosine kinase Syk reduced both TCIPS and TCIPA. Activation of Syk leads to PLCγ2 phosphorylation and activation.48,49 PLCγ2 is a central enzyme in inducing the production of second messengers inositol (1,4,5)-trisphosphate and diacylglycerol leading to raised levels of intracellular calcium and activation of PKC which can positively regulate α- and dense-granule secretion in platelets as well as platelet aggregation.41,50 Inhibition of PLC in our studies significantly reduced aggregation and secretion (Figure 6), indicating that an FcγRIIa–Syk-PLCγ signaling pathway is activated. In contrast, we observed that BAPTA, a calcium chelator, has no significant effect on platelet secretion responses, suggesting that calcium is not required. Importantly, inhibitors of PKC (GF109203X) demonstrated a potent dual inhibition of both TCIPS and TCIPA. Together, these data indicate that signaling for PC3M-luc–mediated dense-granule secretion involves FcγRIIa molecular components by engaging downstream signaling mechanisms including Syk and that this event would be sufficient to initiate dense-granule secretion. Further studies will be necessary to identify the PKC isoform(s) activated during PC3M-luc–induced platelet aggregation and secretion.
The discovery of FcγRIIa as an important mediator of both platelet aggregation and secretion in response to PC3M-luc makes it a strong potential target for therapeutic intervention. This raises the possibility of TCIPS as a target for potential intervention in tumor angiogenesis and metastasis and in tumor-derived thrombosis. The inhibition of this platelet receptor, although not targeting the tumor cell itself, could potentially reduce metastasis and prevent thrombotic events associated with cancer without compromising the hemostatic functions of platelets.
The nature of the cancer cell ligand that stimulates platelet activation remains to be elucidated. Podoplanin is a recently identified protein found on some cancer types that acts as ligand for CLEC-2, a platelet ITAM-containing receptor. However, podoplanin is not usually found on colon or prostate cancer48 and we further confirmed this by flow cytometry analysis (data not shown). In vivo, it is likely that antitumor antibodies could be inadvertent accessories in platelet responses, binding to FcyRIIa via their Fc portion and resulting in enhanced facilitation of tumor cell transport to a metastatic site instead of the intended immune-mediated elimination of a damaging invader cell. However, in the assays performed in this study, we used washed HPs and washed human prostate cancer cells, so no exogenous antibodies are available for activation of platelets. Therefore, an alternative mode for the FcyRIIa receptor must be operating. Recently, in platelets, FcγRIIa has been identified as a transmembrane receptor responsible for mediating “outside-in” signaling through αIIbβ3.51 Of note, we have identified that, in addition to the FcγRIIa, Syk, and PKC axis in platelets, inhibitors of αIIbβ3 had a parallel profile of inhibition of tumor cell–induced platelet activation. Thus, we can propose a collaborative role for the αIIbβ3 and FcγRIIa receptors in recognition of cancer cells by platelets. Subsequent activation and secretion of granule contents may cause a cycle of platelet recruitment and protection of circulating tumor cells that ultimately facilitates tumor cell survival in the circulation and at metastatic sites.
Finally, it is noteworthy that platelet TxA2 synthesis is a critical component of ADP-induced platelet activation. Aspirin, a potent inhibitor of platelet thromboxane production and a widely used antiplatelet agent, has demonstrable potential as an antineoplastic agent.52 In a large metaanalysis of >17 000 patients, daily usage of low-dose aspirin has been shown to reduce the incidence, growth, and metastasis of a number of cancers in a period of 5 to 6 years.52 This finding provides proof of principle for antiplatelet pharmacological intervention to specifically prevent cancer metastasis. Although our studies failed to demonstrate a significant effect of aspirin on TCIPA and TCIPS, a strong tendency toward inhibition was noted. Our data suggest that antiplatelet treatment with P2Y12 inhibitors may provide a superior inhibition of platelet activation in response to tumor cells and this may have a therapeutic implication.
The cell numbers used in this study are admittedly in excess of numbers that might be found physiologically in circulating blood. Circulating tumor cell counts of >30/mL are routinely observed in prostate cancer patients.53 The detection of circulating tumor cells (CTCs) in the blood and disseminated tumor cells (DTCs) in the bone marrow of cancer patients is associated with a poor prognosis.54 Our study demonstrates that platelet activation can occur after a brief, but targeted, interaction with high numbers of cancer cells in vitro. The in vivo correlate of our study would allow for a longer interaction time with CTCs and a more targeted interaction with DTCs. Thus, we believe that although the numbers of cancer cells used in our study are not physiological, the deductions made from such in vitro studies will have physiological relevance if time and opportunity are taken into account.
In conclusion, this work demonstrates for the first time, the capability of cancer cells to directly promote platelet secretion which is required to augment tumor cell–induced platelet aggregation. In addition, we have identified molecular mediators divergently activated in the aggregation and secretion responses (Figure 7). Furthermore and most importantly, we recognize a novel essential role for FcγRIIa in prostate cancer cell–induced platelet activation opening the door to novel antimetastatic therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Michael Berndt (RCSI, Dublin) and Dr Pat Metharom (RCSI, Dublin) for the generous supply of some of the pharmacological antagonists used in this study and for help with mouse platelet studies; Prof Peter Newman (Blood Center, Wisconsin) for generously providing IV.3 Fab(s); Prof Marek Radomsky (TCD, Dublin) and Prof Judy Harmey (RCSI, Dublin) for the kind gifts of Caco-2 and PC3M-luc cells, respectively; and Dr Ann Hopkins (RCSI, Dublin) for providing MCF-7 and MDA-MB231 breast cancer cell lines.
This work was supported by the Health Research Board (HRB) in Ireland under grant PHD/2007/11 with supplementary support from the Department of Molecular and Cellular Therapeutics, Royal College of Surgeons in Ireland.
Authorship
Contribution: A.M. and N.M. conceived the study and designed the research; A.M. performed the experiments and wrote the manuscript; D.W. provided clinical advice; N.M. and D.W. reviewed the manuscript; and S.W.K. provided reagents and methodical advice for all signaling components of this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niamh Moran, School of Postgraduate Study, Molecular and Cellular Therapeutics, Royal College of Surgeons in Ireland, 123 St. Stephens Green, Dublin 2, Ireland; e-mail: nmoran@rcsi.ie.