In this issue of Blood, Choorapoikayil et al have used phosphatase and tensin homolog (Pten)–deficient zebrafish to uncover prominent roles for Pten loss in enhancing proliferation of hematopoietic stem cells (HSCs) and eliciting differentiation arrest within lineage-committed blood progenitor cells. These results provide novel insights into the early genetic events that predispose blood cells to malignant transformation.1
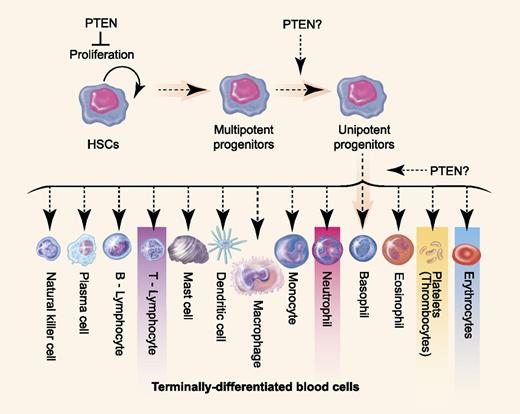
Multiple roles for PTEN in normal blood cell development. PTEN loss would lead to elevated HSC proliferation and to differentiation arrest in lineage-committed progenitor cells. Pten loss leads to reduced numbers of mature erythrocytes, thrombocytes, neutrophils, and thymocytes in the zebrafish model (denoted by boxes). Professional illustration by Paulette Dennis.
Multiple roles for PTEN in normal blood cell development. PTEN loss would lead to elevated HSC proliferation and to differentiation arrest in lineage-committed progenitor cells. Pten loss leads to reduced numbers of mature erythrocytes, thrombocytes, neutrophils, and thymocytes in the zebrafish model (denoted by boxes). Professional illustration by Paulette Dennis.
PTEN is a tumor suppressor gene commonly inactivated in hematopoietic malignancies including T-cell acute lymphoblastic leukemia (T-ALL).2,3 The early events following PTEN inactivation in HSCs and its role in regulating normal blood cell differentiation have not been fully explored.
PTEN is a well-known tumor suppressor that, when lost, leads to constitutive activation of the phosphoinositide 3-kinase (PI3K)/AKT pathway. Pten inactivation is embryonic lethal in mice4 and Drosophila5 ; however, conditional loss within HSCs leads to enhanced proliferation and expanded numbers of HSCs.6,7 Paradoxically, Pten loss results in stem cell exhaustion and reduced ability of HSCs to reconstitute the blood system of irradiated mice. Targeted inactivation of Pten within the HSC compartment also predisposes mice to developing early onset myeloproliferative disease and T-ALL that are fully transplantable. Together, these observations suggest prominent differences in the role Pten plays in regulating self-renewal of HSCs and leukemia propagating cells derived from committed precursors.6 To date, it has been difficult to assess how Pten loss in HSCs is linked with disease onset later in life and how Pten differentially affects HSCs and committed, lineage-restricted blood cell progenitors.
Choorapoikayil and colleagues report that Pten loss leads to enhanced HSC proliferation within the developing zebrafish, but also paradoxically leads to differentiation arrest and expansion of early lineage-committed progenitor cells.1 Pten loss ultimately led to a severe reduction in the numbers of mature thymocytes, thrombocytes, erythrocytes, and neutrophils. Chemical epistasis experiments show that this differentiation arrest can be reversed by LY294002, a potent inhibitor of PI3Ks. Chemical inhibitors restored blood cell maturation even after HSCs colonized and expanded within the caudal hematopoietic tissue, confirming that Pten has important and yet divergent roles within HSCs and early lineage-restricted progenitor cells. These results suggest that PTEN loss likely impacts leukemogenesis by blocking cells in early progenitor cell fates, thereby creating a larger pool of precursor cells in which acquired genetic and/or epigenetic lesions induce frank malignancy.
Given the prominent role PTEN has in expanding the numbers of self-renewing HSCs, it will be important to assess if and how PTEN regulates proliferation in committed, lineage-restricted progenitor cells and leukemia. For example, it is possible that PTEN loss both expands lymphoid progenitor cells and increases the overall frequency of self-renewing leukemia propagating cells. Moreover, the downstream pathways mediated by the PTEN/PI3K/AKT signaling axis to ultimately expand progenitor pools, induce malignancy, and maintain leukemic cells has not been fully defined, especially in T-ALL. The PTEN/PI3K/AKT pathway has many downstream effectors including MYC stability to enhance proliferation, activation of the mammalian target of rapamycin (mTOR) pathway to induce dexamethasone resistance in lymphoid malignancies, and suppressed apoptosis through regulation of BAD and NF-κb.8 Dissecting which of these and other downstream pathways regulate lineage-restricted progenitor cell expansion and leukemic cell maintenance will likely be important for identifying novel therapies for the treatment of a wide range of hematopoietic malignancies. Finally, it will be important to identify the hematopoietic cell stages at which Pten-deficient progenitor cells are arrested (see figure), defining whether the PTEN/PI3K/AKT axis exerts differential effects on multipotent or unipotent progenitor cells. The work from Choorapoikayil et al provides critical insights and a relevant zebrafish model to begin to address these important biological and clinical questions.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal