Key Points
Vaccination with lymphoma cells secreting gp96-Ig together with directed IL-2 rapidly elicit effective tumor immunity after syngeneic HSCT.
IL-2 cytokine-antibody complex expands CD8+ T lymphocytes and NK cells and enhances pathogen immunity early after HSCT.
Abstract
Tumor relapse is the primary cause of mortality in patients with hematologic cancers following autologous hematopoietic stem cell transplantation (HSCT). Vaccination early after HSCT can exploit both the state of lymphopenia and minimal residual disease for generating antitumor immunity. Here, multiple vaccinations using lymphoma cells engineered to secrete heat shock protein fusion gp96-Ig within 2 weeks of T cell-replete syngeneic HSCT led to cross-presentation and increased survival of lymphoma-bearing mice. To enhance vaccine efficacy, interleukin (IL)-2 was directed to predominantly memory phenotype CD8+ T lymphocytes and natural killer (NK) cells via administration bound to anti-IL-2 monoclonal antibody clone S4B6 (IL-2S4B6). Combination therapy with gp96-Ig vaccination and coordinated infusions of IL-2S4B6 resulted in marked prolongation of survival, which directly correlated with ∼500% increase in effector CD8+ T-cell numbers. Notably, this dual regimen elicited large increases in both donor CD8+ T and NK cells, but not CD4+ T lymphocytes; the former 2 populations are essential for both vaccine efficacy and protection against opportunistic infections after HSCT. Indeed, IL-2S4B6-treated HSCT recipients infected with Listeria monocytogenes exhibited decreased bacterial levels. These preclinical studies validate a new strategy particularly well suited to the post-HSCT environment, which may augment adaptive and innate immune function in patients with malignant disease receiving autologous HSCT.
Introduction
Tumor relapse remains the major cause of morbidity and mortality in patients with hematologic malignancies receiving autologous hematopoietic stem cell transplantation (HSCT) for hematolymphoid rescue. According to the Center for International Blood and Marrow Transplant Research, ∼80% of mortality after autologous HSCT (2010-2011) resulted from relapse of primary disease or infection in patients with myeloma, lymphoma, and leukemia.1 Multifaceted immunotherapeutic approaches combined with HSCT for patients with hematopoietic malignancy continue to hold large, but as yet unfulfilled, promise.2 Such enthusiasm for immune-based strategies rests in part from the notion that vaccination regimens can be used early after HSCT during “reboot” of the immune system to promote efficient antitumor and antipathogen immunity by taking advantage of minimal residual disease and the lymphopenia present.3-9 Nevertheless, generating successful protocols early after HSCT must account for the relative dearth of T cells, as well as the need for a vaccine with appropriate tumor or pathogen antigens to promote successful immunity.
Heat shock protein gp96 is the resident endoplasmic reticulum protein chaperone and is intimately involved in MHC-I restricted antigen presentation.10-16 Following necrosis, gp96-peptide complexes are released and can be taken up by antigen presenting cells (APCs), leading to peptide delivery and their efficient activation.17,18 These APCs can therefore cross-present gp96-chaperoned peptides to CD8+ T lymphocytes,19,20 inducing their activation, expansion, and development of effector function. The vaccine used in the present studies consisted of tumor cells engineered to secrete a modified gp96 molecule lacking the endoplasmic reticulum KDEL (Lys-Asp-Glu-Leu) retention signal fused to the FC portion of murine IgG1 (gp96-Ig).21,22 This potent cell vaccine resulted in stimulation of multiple antigen-specific CD8+ T-cell populations in mice (tumor reactive)23-26 and primates (viral reactive),27,28 which prolonged survival in relevant preclinical models of cancer and acute infection, respectively. Moreover, recent studies found the majority of lung cancer patients vaccinated with a gp96-Ig-secreting tumor cell vaccine generated a CD8+ interferon (IFN)-γ+ response (allo-reactive), and these individuals exhibited prolonged survival compared with nonresponders.29 Notably, gp96-Ig vaccination also stimulated natural killer (NK) cells in antitumor models, and this population was hypothesized to contribute to CD8+ T-cell expansion.30
Interleukin (IL)-2 therapy has demonstrated significant antitumor activity in experimental models and has diverse affects following HSCT, in part dependent on dose and time of infusion.31,32 However, because IL-2-induced expansion of T-regulatory cells (Treg) could inhibit antitumor immunity, an important advance for use of this cytokine would be to direct its activity primarily to antitumor effector vs Treg cells.33-35 Notably, recent findings have reported that IL-2 conjugated to a particular anti-IL-2 monoclonal antibody (mAb) can augment antitumor responses.36,37 One cytokine-antibody complex using mAb clone S4B6 (IL-2S4B6), which activates the intermediate affinity IL-2 receptor (β and γ), was found to stimulate the proliferation of predominately memory phenotype CD8+ T lymphocytes and NK cells—2 populations essential for optimal gp96-Ig-induced antitumor responses.30
The preclinical studies presented here investigated the efficacy of vaccination with tumor cells secreting gp96-Ig together with an IL-2S4B6 complex in experimental mouse models of minimal residual lymphoma following syngeneic HSCT. The results obtained support the notion that the effect of gp96-Ig vaccination via cross-presentation early after autologous HSCT was to elicit tumor-reactive CD8+ T cells, and together with directed IL-2 treatment, markedly augmented effector CD8+ T-cell levels. Global expansion of donor CD8+ T lymphocytes and NK cells, but not CD4+ T lymphocytes, following administration of this IL-2S4B6 complex contributed to prolonged survival of lymphoma-bearing HSCT recipients, as well as augmented antipathogen responsiveness early after HSCT.
Materials and methods
Mice
C57BL/6 wild-type (WT) mice (B6, CD45.2+CD90.2+), B6-CD45.1+ and B6-CD90.1+ congenic strains, and B6-CD80−/−CD86−/− double deficient mice (B7KO)38 were obtained from Charles River Laboratories, Taconic Farms, The Jackson Laboratory, or the National Cancer Institute. B6-OT-I mice,39 provided by M. Bevan, were backcrossed onto B6-RAG1−/− background.40 Batf3-deficient (B6-Batf3−/−, Batf3KO)41,42 bone marrow (BM) was provided by the laboratory of P. Reddy via mice from K. Murphy. Perforin-1-deficient mice (B6-Pfp−/−, PrfKO) were generated as described.41 B6-FoxP3mRFP mice42 were provided by R. Flavell. Mice were subsequently bred and maintained under Division of Veterinary Resources supervision and Institutional Animal Care and Use Committee-approved protocols.
Antibodies and staining
Fluorescent antibodies were purchased from BD Biosciences, eBioscience, BioLegend, or Life Technologies and used for flow cytometric analysis: anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-CD11b (M1/70), anti-CD11c (N418), anti-CD19 (6D5), anti-CD25 (PC61), anti-CD44 (IM7), anti-CD45.1 (A20), anti-CD62L (MEL-14), anti-CD90.1 (OX-7), anti-CD122 (TM-β1), anti-CD127 (A7R34), anti-F4/80 (BM8), anti-Gr-1 (RB6-8C5), anti-IFN-γ (XMG1.2), anti-killer cell lectin-like receptor subfamily G member 1 (KLRG-1) (2F1), anti-NK1.1 (PK136), anti-tumor necrosis factor (TNF)-α (MP6-XT22), anti-Vα2 (B20.1), and anti-Vβ5.1/5.2 (MR9-4). To enumerate T-cell receptor (TCR) ovalbumin (OVA)257-264 (SIINFEKL)-reactive CD8+ T cells, 0.5 μg DimerX−Kb (BD Biosciences) was incubated with 0.33 μg OVA257-264 overnight. For intracellular cytokine staining, single cell suspensions prepared from tissues were incubated (1.0 × 106 cells/mL) in 10% complete medium with 0.1 nM OVA257-264 for 4 to 6 h at 37°C, with monensin (GolgiStop; BD Biosciences) or brefeldin A (GolgiPlug; BD Biosciences), surface stained, fixed, and permeabilized with the FoxP3 staining kit (eBioscience) overnight, stained intracellularly, and analyzed. Samples were acquired on a BD-LSRFortessa, LSRII, or Accuri-C6 flow cytometer and analyzed with BD-FACSDiva or CFlow software.
Tumor cell lines
EL-4 lymphoma cells43 expressing OVA (E.G7),44 provided by M. Bevan, were engineered to secrete gp96-Ig (E.G7-gp96-Ig and EL4-gp96-Ig) as previously described.21 Cell lines were maintained in Iscove's modified Dulbecco's medium containing 10% fetal bovine serum, 1 μg/mL gentamicin, 0.05 mmol/L β-mercaptoethanol, and the appropriate antibiotics: G418 (0.4 mg/mL) or l-histidinol (2 mmol/L).
Preparation of donor T-cell populations
CD8+ T cells specific for OVA257-264 (OT-I) were purified by negative selection from the spleen and lymph nodes (LNs) of B6-OT-I (RAG1−/−) mice with the CD8+ T Cell Isolation Kit using magnetic separation (≥95% by flow cytometry; Miltenyi Biotec). Polyclonal CD4+ and CD8+ T cells were enriched (≥75%) following B-cell depletion (≥90%) from the spleen and LNs of donor mice using plate-bound goat anti-mouse IgG/M as previously described (Millipore).45,46 Polyclonal CD4+ (≥88%) and CD8+ (≥90%) T cells were purified by negative selection following B-cell depletion.
BM transplantation
BM was isolated from femurs, tibias, and vertebral columns as previously described.45,46 Briefly, T cells were removed by incubation with anti-CD90.2 (HO134), anti-CD4 (RL174), and anti-CD8 (H022) ascites (1:5) with 12% (v/v) Low-Tox M rabbit complement (Cedarlane Labs) at a final concentration of 25.0 × 106 cells/mL on ice for 15 minutes, followed by 37°C incubation for 30 minutes. B6 mice were conditioned with 9.5-Gy total body irradiation. The following day, 5.0 × 106 congenic B6-CD45.1+ T cell-depleted (TCD) BM cells were infused intravenously alone or together with the selected donor T-cell populations (0.2 mL RPMI 1640). Mice were maintained briefly on gentamicin-supplemented water.
Tumor cell inoculation into BM recipients or T-cell donors
E.G7 and EL-4 lymphoma cells were isolated from log-phase cultures. One day following BM transplants, 1.0 × 105 or 5000 cells, respectively, were injected intraperitoneally (0.5 mL RPMI 1640). Tumor-bearing T-cell donor mice were inoculated intraperitoneally with 4.0 × 106 E.G7 cells 3 weeks before T-cell harvest. Some donor mice were injected intravenously with 5.0 × 105 negatively selected CD8+ OT-I T cells 1 day earlier. For subcutaneous challenge experiments, 1 × 106 cells were inoculated (0.1 mL RPMI 1640).
Vaccination
E.G7-gp96-Ig and EL4-gp96-Ig cells were isolated from log-phase cultures. Two or 3 days after adoptive transfer of T cells, 1.0 × 107 irradiated (40-120 Gy) cells were injected intraperitoneally (0.5 mL RPMI 1640). Vaccinations were repeated on subsequent days as indicated.
IL-2 cytokine-antibody complex
Recombinant mouse IL-2 (1.5 μg; eBioscience) was incubated with 8 μg anti-mouse IL-2 mAb clone S4B6 (BD Biosciences) for 15 minutes at room temperature (IL-2S4B6) as previously described.33 IL-2 complexes were injected intraperitoneally (0.5 mL phosphate-buffered saline).
Bacterial infection and determination of colony-forming units
Recombinant Listeria monocytogenes expressing OVA134-387,47,48 provided by H. Chen, was grown in brain/heart infusion broth. Log-phase growing bacteria (OD560 0.2) were diluted (phosphate-buffered saline) to 1.0 × 104 colony-forming units (CFUs) and injected intravenously (0.2 mL Hank's balanced salt solution). The number of bacteria injected was confirmed by growth on brain/heart infusion agar plates. Seven days after infection, spleens were disrupted using 0.2-μm screens in 0.05% Triton X-100, and CFUs were determined by serial dilutions after incubation for 18 hours at 37°C on brain/heart infusion agar plates.
Statistical analysis
Paired comparisons were performed using Student t test, multiple analyses were performed using 1-way or 2-way ANOVA, and survival analyses were performed using the log-rank test: *P ≤ .05, **P ≤ .01, ***P ≤ .001, and ****P ≤ .0001.
Results
CD8+ T-cell response elicited following vaccination with tumor cells secreting gp96-Ig in syngeneic HSCT recipients
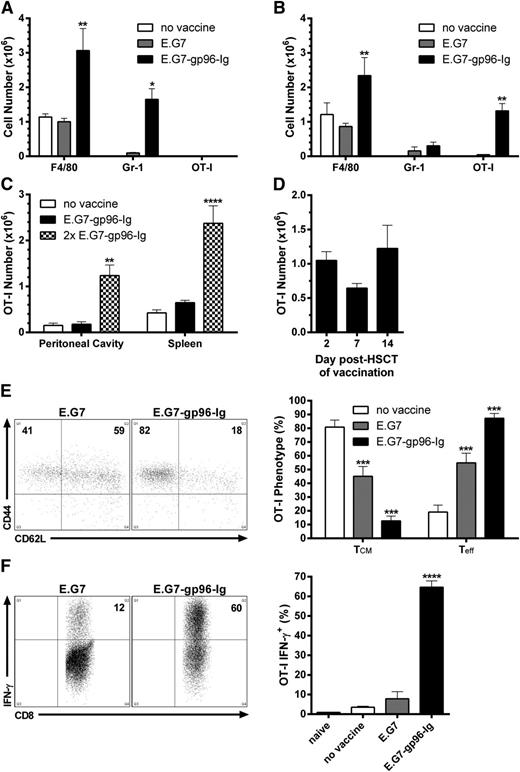
To assess the immune consequences of administering gp96-Ig-secreting tumor cells early following autologous HSCT, B6 mice were conditioned (9.5 Gy) and transplanted with B6-CD45.1+ TCD BM cells. To monitor a tumor-reactive CD8+ T-cell population, TCD BM was supplemented with 1.0 × 106 CD8+ T cells specific for OVA257-264 (OT-I). Following a single intraperitoneal vaccination with 1.0 × 107 EL-4 lymphoma cells (120 Gy) expressing OVA (E.G7)-secreting gp96-Ig (E.G7-gp96-Ig), large numbers of macrophages (F4/80hi CD11bhi Gr-1lo) and inflammatory monocytes (Gr-1hi CD11bmid F4/80lo)49,50 rapidly accumulated at the vaccine site (2 days after vaccination), comprising ∼90% of its entire cellularity (Figure 1A). Initially, these myeloid cells were of recipient origin, but were rapidly replaced by their donor counterparts over the course of the immune response (within days, data not shown). Interestingly, at 2 days after vaccination, tumor-reactive CD8+ T lymphocytes could not be detected at the vaccine site (Figure 1A); however, within 24 hours, elevated levels were observed in the blood (data not shown), and maximum numbers were reached in the peritoneal cavity 5 days following a single vaccination (Figure 1B). At this time, large numbers of macrophages could still be enumerated, together with lower numbers of inflammatory monocytes (Figure 1B). Notably, vaccination with parental E.G7 cells containing endogenous gp96 did not induce these cellular responses (Figure 1A-B).
Vaccination with tumor cells secreting the heat shock protein fusion gp96-Ig led to the activation, expansion, and functional competence of tumor-reactive CD8+ T lymphocytes in syngeneic HSCT recipients. Conditioned (9.5 Gy) B6 mice were transplanted with 5.0 × 106 B6-CD45.1+ TCD-BM cells and adoptively transferred with 1.0 × 106 CD8+ T cells specific for OVA257-264 (OT-I) 5 days later. After a 2-day resting period, recipients were vaccinated intraperitoneally with irradiated (120 Gy) EL-4 lymphoma cells expressing OVA (E.G7) engineered to secrete gp96-Ig (E.G7-gp96-Ig). (A) Macrophages (F4/80hi CD11bhi Gr-1lo) and inflammatory monocytes (Gr-1hi CD11bmid F4/80lo) infiltrated the peritoneal cavity 2 days following vaccination with gp96-Ig-secreting tumor cells; n = 9 from a pool of 3 experiments. (B) Tumor-reactive CD8+ T lymphocytes (OT-I: CD8+ CD45.1− Vα2+ Vβ5+) expanded in the peritoneal cavity 5 days following vaccination with tumor cells secreting gp96-Ig; n = 8 from a pool of 3 experiments. (C) Tumor-reactive CD8+ T cells accumulated in secondary lymphoid organs 5 days following a second vaccination (13 days after HSCT) with gp96-Ig-secreting tumor cells; n = 3. (D) Vaccination with gp96-Ig-secreting tumor cells up to 2 weeks after HSCT resulted in expansion of cotransplanted tumor-reactive CD8+ T cells 5 days later; n = 3 to 8 from 3 independent experiments. (E) Tumor-reactive CD8+ T lymphocytes transitioned from central-memory (TCM, CD62L+ CD44+) to effector cells (Teff, CD62L− CD44+) following vaccination with tumor cells secreting gp96-Ig: (left) representative dot plots; (right) n = 5 to 6 from a pool of 2 experiments. (F) Vaccination with gp96-Ig-secreting tumor cells induced highly IFN-γ+ tumor-reactive CD8+ T cells: (left) representative dot plots; (right) n = 4 from a representative of 3 experiments.
Vaccination with tumor cells secreting the heat shock protein fusion gp96-Ig led to the activation, expansion, and functional competence of tumor-reactive CD8+ T lymphocytes in syngeneic HSCT recipients. Conditioned (9.5 Gy) B6 mice were transplanted with 5.0 × 106 B6-CD45.1+ TCD-BM cells and adoptively transferred with 1.0 × 106 CD8+ T cells specific for OVA257-264 (OT-I) 5 days later. After a 2-day resting period, recipients were vaccinated intraperitoneally with irradiated (120 Gy) EL-4 lymphoma cells expressing OVA (E.G7) engineered to secrete gp96-Ig (E.G7-gp96-Ig). (A) Macrophages (F4/80hi CD11bhi Gr-1lo) and inflammatory monocytes (Gr-1hi CD11bmid F4/80lo) infiltrated the peritoneal cavity 2 days following vaccination with gp96-Ig-secreting tumor cells; n = 9 from a pool of 3 experiments. (B) Tumor-reactive CD8+ T lymphocytes (OT-I: CD8+ CD45.1− Vα2+ Vβ5+) expanded in the peritoneal cavity 5 days following vaccination with tumor cells secreting gp96-Ig; n = 8 from a pool of 3 experiments. (C) Tumor-reactive CD8+ T cells accumulated in secondary lymphoid organs 5 days following a second vaccination (13 days after HSCT) with gp96-Ig-secreting tumor cells; n = 3. (D) Vaccination with gp96-Ig-secreting tumor cells up to 2 weeks after HSCT resulted in expansion of cotransplanted tumor-reactive CD8+ T cells 5 days later; n = 3 to 8 from 3 independent experiments. (E) Tumor-reactive CD8+ T lymphocytes transitioned from central-memory (TCM, CD62L+ CD44+) to effector cells (Teff, CD62L− CD44+) following vaccination with tumor cells secreting gp96-Ig: (left) representative dot plots; (right) n = 5 to 6 from a pool of 2 experiments. (F) Vaccination with gp96-Ig-secreting tumor cells induced highly IFN-γ+ tumor-reactive CD8+ T cells: (left) representative dot plots; (right) n = 4 from a representative of 3 experiments.
Syngeneic HSCT recipients vaccinated a second time (6 days following the first vaccination) with tumor cells secreting gp96-Ig contained similar numbers of tumor-reactive CD8+ T cells at the vaccine site as observed after a single vaccination, and elevated numbers of these cells were now detected in the spleen (Figure 1C). Proliferative signals engendered by lymphopenia diminish over time in myeloablatively conditioned mice.8,51 We found that vaccination could be delayed 1 to 2 weeks after HSCT, and similar levels of tumor-reactive CD8+ T lymphocytes were observed at the vaccine site 5 days following a single vaccination (Figure 1D). CD8+ T cells introduced into a lymphopenic environment assume a central-memory phenotype (CD62L+CD44+; Figure 1E).52-56 Following a single vaccination with gp96-Ig-secreting tumor cells, ≥80% of tumor-reactive CD8+ T cells transitioned to effector cells (CD62L−CD44+; Figure 1E). Peptide stimulation of these cells ex vivo corroborated this effector phenotype because ≥60% expressed IFN-γ (Figure 1F). In nontransplanted mice, gp96 has been reported not to induce stimulation of CD4+ T cells,11,21 and we detected no appreciable tumor-reactive (OVA323-339) CD4+ T-cell (OT-II) expansion following vaccination with tumor cells secreting gp96-Ig in syngeneic HSCT recipients (data not shown).
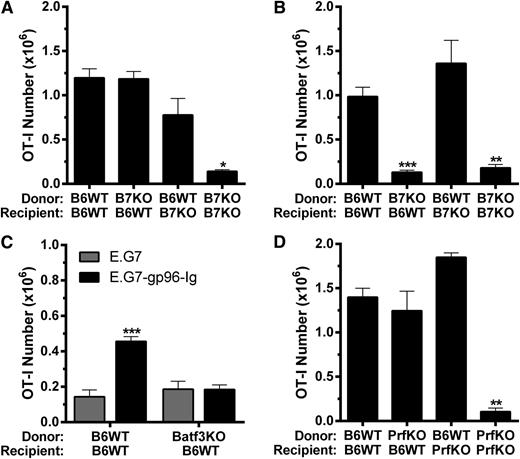
To determine if B7 costimulation was required for antigen-specific CD8+ T-cell expansion induced by gp96-Ig vaccination in HSCT recipients, as well as the source of this signal regarding APCs, we used donor or recipient mice deficient in CD80/CD86 (B7KO). When vaccine was administered 2 days after HSCT, donor and recipient APCs were both found to contribute to overall tumor-reactive CD8+ T-lymphocyte expansion (Figure 2A). Strikingly, when vaccination was delayed 1 week after HSCT, only donor APCs contributed to the response, because such expansion was not observed when B7-deficient BM cells were transplanted into WT hosts (Figure 2B). To address the importance of cross-presentation by these donor APCs, TCD BM from Batf3-deficient mice (Batf3KO), which lack CD11c+CD8α+ cross-presenting dendritic cells,57,58 was used for transplantation (supplemental Figure 1). These recipients were unable to expand tumor-reactive CD8+ T cells following gp96-Ig vaccination (Figure 2C). Our group reported that perforin-1 was required for tumor-reactive CD8+ T-lymphocyte expansion induced by vaccination with tumor cells secreting gp96-Ig in nontransplanted mice.30 Using either donors or recipients deficient in perforin-1 (PrfKO), transplants determined that perforin-1 was also required in HSCT recipients, and interestingly, donor- or recipient-derived perforin-1 was sufficient to support this expansion (Figure 2D).
B7 costimulation, Batf3 and perforin-1 were required for optimal tumor-reactive CD8+ T-lymphocyte expansion induced by vaccination with gp96-Ig-secreting tumor cells following syngeneic HSCT. BM transplants were performed as in Figure 1 using mice deficient in (A-B) CD80 and CD86 (B7KO), (C) Batf3 (Batf3KO), or (D) perforin-1 (PrfKO) as donors and/or recipients with (A) coinfusion or (B-D) delayed infusion of (A-B,D) 1.0 × 106 or (C) 0.5 × 106 tumor-reactive CD8+ T cells (OT-I), and the peritoneal cavity was analyzed 5 days following intraperitoneal vaccination with tumor cells secreting gp96-Ig. (A) Donor and recipient APC contributed to expansion of tumor-reactive CD8+ T cells, that were cotransplanted with the BM, following vaccination with gp96-Ig-secreting tumor cells; n = 3 to 9 from a pool of 3 experiments. (B) Only donor APC contributed to expansion of tumor-reactive CD8+ T lymphocytes, that were infused 5 days after HSCT, induced by vaccination with tumor cells secreting gp96-Ig; n = 2 to 4 from a pool of 2 experiments. (C) Donor cross-presenting CD11c+ CD8α+ dendritic cells were required for optimal expansion of tumor-reactive CD8+ T cells following vaccination with gp96-Ig-secreting tumor cells; n = 5. (D) Perforin-1 could be supplied by either donor or recipient cells for optimal expansion of tumor-reactive CD8+ T lymphocytes following vaccination with tumor cells secreting gp96-Ig; n = 2 to 4 from a pool of 2 experiments.
B7 costimulation, Batf3 and perforin-1 were required for optimal tumor-reactive CD8+ T-lymphocyte expansion induced by vaccination with gp96-Ig-secreting tumor cells following syngeneic HSCT. BM transplants were performed as in Figure 1 using mice deficient in (A-B) CD80 and CD86 (B7KO), (C) Batf3 (Batf3KO), or (D) perforin-1 (PrfKO) as donors and/or recipients with (A) coinfusion or (B-D) delayed infusion of (A-B,D) 1.0 × 106 or (C) 0.5 × 106 tumor-reactive CD8+ T cells (OT-I), and the peritoneal cavity was analyzed 5 days following intraperitoneal vaccination with tumor cells secreting gp96-Ig. (A) Donor and recipient APC contributed to expansion of tumor-reactive CD8+ T cells, that were cotransplanted with the BM, following vaccination with gp96-Ig-secreting tumor cells; n = 3 to 9 from a pool of 3 experiments. (B) Only donor APC contributed to expansion of tumor-reactive CD8+ T lymphocytes, that were infused 5 days after HSCT, induced by vaccination with tumor cells secreting gp96-Ig; n = 2 to 4 from a pool of 2 experiments. (C) Donor cross-presenting CD11c+ CD8α+ dendritic cells were required for optimal expansion of tumor-reactive CD8+ T cells following vaccination with gp96-Ig-secreting tumor cells; n = 5. (D) Perforin-1 could be supplied by either donor or recipient cells for optimal expansion of tumor-reactive CD8+ T lymphocytes following vaccination with tumor cells secreting gp96-Ig; n = 2 to 4 from a pool of 2 experiments.
Effectiveness of gp96-Ig-secreting tumor cell vaccination in a preclinical minimal residual lymphoma syngeneic HSCT model
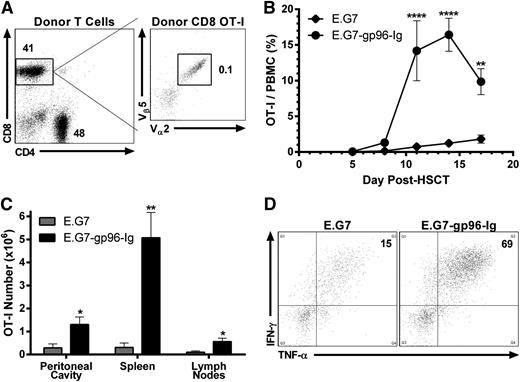
As occurs in clinical transplants, T cells were obtained from donors bearing tumor. TCD BM (Figure 1) was supplemented with 2.0 × 106 enriched CD4+ and CD8+ T lymphocytes from tumor-bearing B6-CD90.1+ donors. To enable monitoring, some tumor bearing T-cell donors were infused intravenously with 5.0 × 105 CD8+ OT-I T cells 1 day preceding tumor inoculation. Based on TCR Vαβ analysis, we calculated that ∼1000 transgenic tumor-reactive CD8+ T lymphocytes were included in the 2.0 × 106 donor T-cell inoculum when such tumor-bearing animals were used (Figure 3A). Two days after HSCT, B6 recipients were vaccinated intraperitoneally with 1.0 × 107 gp96-Ig-secreting tumor cells (40 Gy) and repeated every 3 days (total = 5 vaccinations). Rapid and potent tumor-reactive CD8+ T-lymphocyte expansion was identified within the first 3 weeks after HSCT (Figure 3B). Five days following the third vaccination (at the peak of the response; Figure 3B), tumor-reactive CD8+ T cells accumulated at the vaccine site (>1000× the input number), spleen (>5000×), and draining LNs (>500×; Figure 3C). Following peptide stimulation ex vivo, a majority of these lymphocytes from the vaccine site were polyfunctional,59 coproducing IFN-γ and TNF-α (Figure 3D). Repeated vaccinations with parental E.G7 cells again failed to induce appreciable expansion or responsiveness above that resulting from lymphopenia alone (Figure 3B-D).
Tumor-reactive CD8+ T lymphocytes obtained from tumor-bearing donors were expanded and functional following transplantation into syngeneic HSCT recipients and vaccination with tumor cells secreting gp96-Ig. Conditioned (9.5 Gy) B6 recipients received B6-CD45.1+ TCD-BM cells supplemented with 2.0 × 106 B6-CD90.1+ CD4+ and CD8+ T lymphocytes obtained from E.G7 lymphoma-bearing donors, containing ∼1000 tumor-reactive CD8+ T cells (OT-I). Recipients were vaccinated intraperitoneally with irradiated (40 Gy) E.G7 cells secreting gp96-Ig 2 days after HSCT and repeated every 3 days for a total of 5 vaccinations. (A) CD4+ and CD8+ splenic and LN T cells from a typical tumor-bearing B6-CD90.1+ donor mouse. These animals were injected 3 weeks before HSCT with 5.0 × 105 CD8+ OT-I T lymphocytes intravenously and 4.0 × 106 E.G7 lymphoma cells intraperitoneally and bear progressively growing tumors. Tumor was only detectable at the site of injection (peritoneal cavity) at this time. Total events analyzed represented 2.5 × 106 viable cells, and CD8+ OT-I T lymphocytes were clearly identified (1000 CD8+ CD90.1− Vα2+ Vβ5+/1.0 × 106 CD8+ CD90.1+). (B) Multiple vaccinations with gp96-Ig-secreting tumor cells induced expansion of tumor-reactive CD8+ T cells obtained from tumor bearing donors; n = 4; ♦, E.G7; ●, E.G7-gp96-Ig. (C) Tumor-reactive CD8+ T lymphocytes expanded at the vaccine site and other lymphoid tissues 5 days following 3 vaccinations with tumor cells secreting gp96-Ig; n = 2. (D) Vaccination with gp96-Ig-secreting tumor cells induced highly IFN-γ+ and TNF-α+ tumor-reactive CD8+ T cells 5 days following 3 vaccinations; representative dot plots from n = 2.
Tumor-reactive CD8+ T lymphocytes obtained from tumor-bearing donors were expanded and functional following transplantation into syngeneic HSCT recipients and vaccination with tumor cells secreting gp96-Ig. Conditioned (9.5 Gy) B6 recipients received B6-CD45.1+ TCD-BM cells supplemented with 2.0 × 106 B6-CD90.1+ CD4+ and CD8+ T lymphocytes obtained from E.G7 lymphoma-bearing donors, containing ∼1000 tumor-reactive CD8+ T cells (OT-I). Recipients were vaccinated intraperitoneally with irradiated (40 Gy) E.G7 cells secreting gp96-Ig 2 days after HSCT and repeated every 3 days for a total of 5 vaccinations. (A) CD4+ and CD8+ splenic and LN T cells from a typical tumor-bearing B6-CD90.1+ donor mouse. These animals were injected 3 weeks before HSCT with 5.0 × 105 CD8+ OT-I T lymphocytes intravenously and 4.0 × 106 E.G7 lymphoma cells intraperitoneally and bear progressively growing tumors. Tumor was only detectable at the site of injection (peritoneal cavity) at this time. Total events analyzed represented 2.5 × 106 viable cells, and CD8+ OT-I T lymphocytes were clearly identified (1000 CD8+ CD90.1− Vα2+ Vβ5+/1.0 × 106 CD8+ CD90.1+). (B) Multiple vaccinations with gp96-Ig-secreting tumor cells induced expansion of tumor-reactive CD8+ T cells obtained from tumor bearing donors; n = 4; ♦, E.G7; ●, E.G7-gp96-Ig. (C) Tumor-reactive CD8+ T lymphocytes expanded at the vaccine site and other lymphoid tissues 5 days following 3 vaccinations with tumor cells secreting gp96-Ig; n = 2. (D) Vaccination with gp96-Ig-secreting tumor cells induced highly IFN-γ+ and TNF-α+ tumor-reactive CD8+ T cells 5 days following 3 vaccinations; representative dot plots from n = 2.
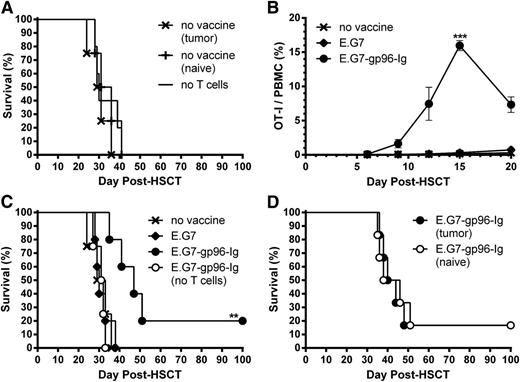
Next, we developed a minimal residual lymphoma model in which recipients were inoculated intraperitoneally with 1.0 × 105 viable E.G7 lymphoma cells 1 day following HSCT, simulating tumor relapse after transplant. This resulted in 100% lethality with a median survival time (MST) of 1 month, regardless of donor T-cell source (tumor-naive or tumor-bearing donors; Figure 4A). In this minimal residual lymphoma model, tumor-reactive CD8+ T-cell expansion was again observed following multiple gp96-Ig vaccinations (Figure 4B). These responses led to a significant MST extension and increased overall survival (20% survived >100 days after HSCT; Figure 4C). Enhanced MST was dependent on transplant of donor T cells because vaccination failed to prolong survival in recipients lacking donor T cells (Figure 4C). Again, vaccination with parental E.G7 cells failed to affect expansion or survival (Figure 4B-C) and the source of donor T cells (naive or tumor-bearing donors) did not change the survival advantage engendered by gp96-Ig vaccination (Figure 4D).
Multiple vaccinations with tumor cells secreting gp96-Ig expanded tumor-reactive CD8+ T lymphocytes and increased survival in T cell-replete syngeneic HSCT recipients with lymphoma. Transplants were performed as in Figure 3, and recipients were inoculated intraperitoneally with 1.0 × 105 E.G7 lymphoma cells the following day to simulate tumor relapse after HSCT. (A) Equivalent survival of HSCT recipients with lymphoma receiving T cells from tumor-bearing or tumor-naive donors or no T cells; n = 4 to 5 from 3 independent experiments; x, no vaccine (tumor); +, no vaccine (naive); −, no T cells. (B) Multiple vaccinations with gp96-Ig-secreting tumor cells efficiently expanded tumor-reactive CD8+ T cells in lymphoma-bearing HSCT recipients. Two days following tumor inoculation, recipients were vaccinated intraperitoneally with irradiated (40 Gy) E.G7 lymphoma cells secreting gp96-Ig and repeated every 3 days for a total of 5 vaccinations; n = 20 from pool of 4 experiments; x, no vaccine; ♦, E.G7; ●, E.G7-gp96-Ig. (C) Vaccination with tumor cells secreting gp96-Ig led to increased MST and overall survival of lymphoma-bearing HSCT recipients; n = 4 to 5; x, no vaccine; ♦, E.G7; ●, E.G7-gp96-Ig; ○, E.G7-gp96-Ig (no T cells). The no vaccine group illustrated here is shown in A with other nonvaccinated groups. (D) Equivalent survival of vaccinated HSCT recipients receiving T cells from tumor-bearing or tumor-naive donors; n = 6; ●, E.G7-gp96-Ig (tumor); ○, E.G7-gp96-Ig (naive).
Multiple vaccinations with tumor cells secreting gp96-Ig expanded tumor-reactive CD8+ T lymphocytes and increased survival in T cell-replete syngeneic HSCT recipients with lymphoma. Transplants were performed as in Figure 3, and recipients were inoculated intraperitoneally with 1.0 × 105 E.G7 lymphoma cells the following day to simulate tumor relapse after HSCT. (A) Equivalent survival of HSCT recipients with lymphoma receiving T cells from tumor-bearing or tumor-naive donors or no T cells; n = 4 to 5 from 3 independent experiments; x, no vaccine (tumor); +, no vaccine (naive); −, no T cells. (B) Multiple vaccinations with gp96-Ig-secreting tumor cells efficiently expanded tumor-reactive CD8+ T cells in lymphoma-bearing HSCT recipients. Two days following tumor inoculation, recipients were vaccinated intraperitoneally with irradiated (40 Gy) E.G7 lymphoma cells secreting gp96-Ig and repeated every 3 days for a total of 5 vaccinations; n = 20 from pool of 4 experiments; x, no vaccine; ♦, E.G7; ●, E.G7-gp96-Ig. (C) Vaccination with tumor cells secreting gp96-Ig led to increased MST and overall survival of lymphoma-bearing HSCT recipients; n = 4 to 5; x, no vaccine; ♦, E.G7; ●, E.G7-gp96-Ig; ○, E.G7-gp96-Ig (no T cells). The no vaccine group illustrated here is shown in A with other nonvaccinated groups. (D) Equivalent survival of vaccinated HSCT recipients receiving T cells from tumor-bearing or tumor-naive donors; n = 6; ●, E.G7-gp96-Ig (tumor); ○, E.G7-gp96-Ig (naive).
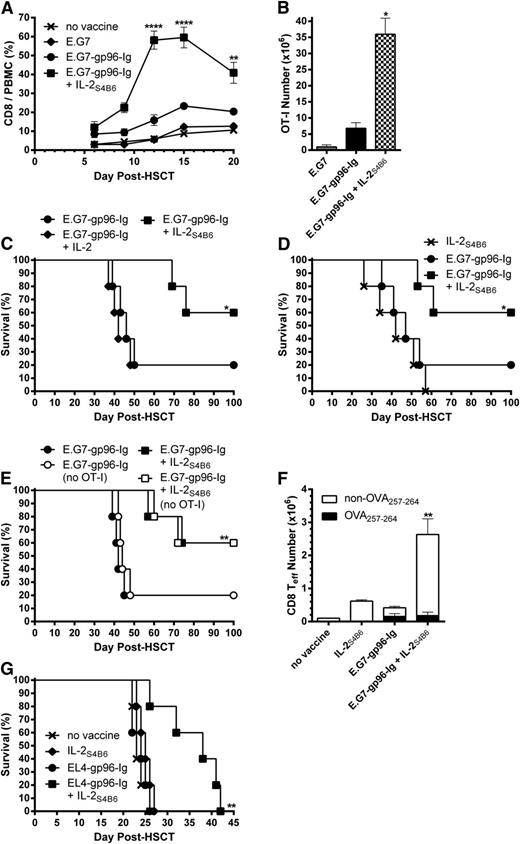
Directed IL-2 augmented CD8+ T-lymphocyte expansion and markedly enhanced survival of lymphoma-bearing HSCT recipients
To strengthen tumor responses elicited by vaccination with gp96-Ig-secreting tumor cells, IL-2 was introduced following vaccination. Recipient mice (Figure 4) were infused intraperitoneally with IL-2S4B6 1 day following each vaccination. This combination treatment potently enhanced total CD8+ T-lymphocyte expansion in the peripheral blood and in tissues of HSCT recipients (Figure 5A; data not shown). However, IL-2S4B6 marginally affected overall CD4+ T-lymphocyte (supplemental Figure 2, left) and CD4+ FoxP3+ Treg levels (supplemental Figure 2, right). Analysis of the vaccine site, spleen, and LN compartments (at the peak of the response; supplemental Figure 3) following vaccination in combination with IL-2S4B6 demonstrated that >30 000× expansion of tumor-reactive CD8+ T lymphocytes had occurred over the input number of ∼1000 (Figure 5B). This combinatorial regimen (gp96-Ig vaccination and IL-2S4B6) resulted in a striking increase in MST (>100 days after HSCT) and overall survival (60%; Figure 5C). In contrast, the same dose of unbound IL-2 (not complexed to mAb) in combination with vaccination exhibited only a limited effect on overall CD8+ T-lymphocyte expansion (supplemental Figure 4, left) and tumor-reactive CD8+ T-cell levels (supplemental Figure 4, right). This resulted in no increase in MST or overall survival compared with vaccination alone (Figure 5C). Furthermore, IL-2S4B6 must be combined with vaccination because, although monotherapy with IL-2S4B6 induced strong total CD8+ T-cell expansion (supplemental Figure 5, left), it only elicited a small response by tumor-reactive CD8+ T lymphocytes (supplemental Figure 5, right). This resulted in no distinct effects on MST or overall survival compared with vaccination alone (Figure 5D). Consistent with a vaccine requirement, IL-2S4B6 treatment in combination with parental E.G7 cells not secreting gp96-Ig (weak vaccine) elicited only a modest response by tumor-reactive CD8+ T cells (supplemental Figure 5, right).
IL-2 administered in vivo after complex with anti-IL-2 mAb effectively expanded CD8+ T lymphocytes and augmented antitumor immunity induced by vaccination with tumor cells secreting gp96-Ig in lymphoma-bearing syngeneic HSCT recipients. Transplants, tumor inoculation, and treatments were performed as in Figure 4, and mice received IL-2S4B6 1 day following each vaccination as indicated. (A) IL-2S4B6 treatment induced robust expansion of CD8+ T cells in vaccinated HSCT recipients with lymphoma during the first 3 weeks after HSCT. CD8+ T-cell frequency in the peripheral blood; n = 20 from a pool of 4 experiments; x, no vaccine; ♦, E.G7; ●, E.G7-gp96-Ig; ■, E.G7-gp96-Ig + IL-2S4B6. (B) Tumor-reactive CD8+ T lymphocytes markedly expanded at the vaccine site and other lymphoid tissues of lymphoma-bearing HSCT recipients 5 days following 3 treatments with IL-2S4B6 and gp96-Ig vaccine. Tumor-reactive CD8+ T-cell number in the peritoneal cavity, spleen, and draining LNs; n = 2. (C) Combination therapy with vaccination and IL-2S4B6 increased MST and overall survival of syngeneic HSCT recipients with lymphoma. Noncomplexed, unbound IL-2 in combination with vaccination failed to enhance survival of lymphoma-bearing HSCT recipients; n = 5; ●, E.G7-gp96-Ig; ♦, E.G7-gp96-Ig + IL-2; ■, E.G7-gp96-Ig + IL-2S4B6. (D) IL-2S4B6 in the absence of vaccination marginally enhanced survival of lymphoma bearing HSCT recipients; n = 5; x, IL-2S4B6; ●, E.G7-gp96-Ig; ■, E.G7-gp96-Ig + IL-2S4B6. (E) MST and overall survival of T cell-replete syngeneic HSCT recipients with lymphoma following vaccination and IL-2S4B6 was independent of transgenic antigen-specific CD8+ T-cell presence; n = 5; ●, E.G7-gp96-Ig; ○, E.G7-gp96-Ig (no OT-I); ■, E.G7-gp96-Ig + IL-2S4B6; □, E.G7-gp96-Ig + IL-2S4B6 (no OT-I). (F) CD8+ effector T-cell numbers at the vaccine site were markedly increased only following vaccine and IL-2S4B6 treatment. CD8+ Teff (CD62L− CD44+), including endogenous non-TCR transgenic DimerX−Kb−OVA257-264+, cell numbers in the peritoneal cavity; n = 5. (G) Combined EL4-gp96-Ig and IL-2S4B6 vaccination strategy prolonged MST in EL4-bearing HSCT recipients; n = 5; x, no vaccine; ♦, IL-2S4B6; ●, EL4-gp96-Ig; ■, EL4-gp96-Ig + IL-2S4B6.
IL-2 administered in vivo after complex with anti-IL-2 mAb effectively expanded CD8+ T lymphocytes and augmented antitumor immunity induced by vaccination with tumor cells secreting gp96-Ig in lymphoma-bearing syngeneic HSCT recipients. Transplants, tumor inoculation, and treatments were performed as in Figure 4, and mice received IL-2S4B6 1 day following each vaccination as indicated. (A) IL-2S4B6 treatment induced robust expansion of CD8+ T cells in vaccinated HSCT recipients with lymphoma during the first 3 weeks after HSCT. CD8+ T-cell frequency in the peripheral blood; n = 20 from a pool of 4 experiments; x, no vaccine; ♦, E.G7; ●, E.G7-gp96-Ig; ■, E.G7-gp96-Ig + IL-2S4B6. (B) Tumor-reactive CD8+ T lymphocytes markedly expanded at the vaccine site and other lymphoid tissues of lymphoma-bearing HSCT recipients 5 days following 3 treatments with IL-2S4B6 and gp96-Ig vaccine. Tumor-reactive CD8+ T-cell number in the peritoneal cavity, spleen, and draining LNs; n = 2. (C) Combination therapy with vaccination and IL-2S4B6 increased MST and overall survival of syngeneic HSCT recipients with lymphoma. Noncomplexed, unbound IL-2 in combination with vaccination failed to enhance survival of lymphoma-bearing HSCT recipients; n = 5; ●, E.G7-gp96-Ig; ♦, E.G7-gp96-Ig + IL-2; ■, E.G7-gp96-Ig + IL-2S4B6. (D) IL-2S4B6 in the absence of vaccination marginally enhanced survival of lymphoma bearing HSCT recipients; n = 5; x, IL-2S4B6; ●, E.G7-gp96-Ig; ■, E.G7-gp96-Ig + IL-2S4B6. (E) MST and overall survival of T cell-replete syngeneic HSCT recipients with lymphoma following vaccination and IL-2S4B6 was independent of transgenic antigen-specific CD8+ T-cell presence; n = 5; ●, E.G7-gp96-Ig; ○, E.G7-gp96-Ig (no OT-I); ■, E.G7-gp96-Ig + IL-2S4B6; □, E.G7-gp96-Ig + IL-2S4B6 (no OT-I). (F) CD8+ effector T-cell numbers at the vaccine site were markedly increased only following vaccine and IL-2S4B6 treatment. CD8+ Teff (CD62L− CD44+), including endogenous non-TCR transgenic DimerX−Kb−OVA257-264+, cell numbers in the peritoneal cavity; n = 5. (G) Combined EL4-gp96-Ig and IL-2S4B6 vaccination strategy prolonged MST in EL4-bearing HSCT recipients; n = 5; x, no vaccine; ♦, IL-2S4B6; ●, EL4-gp96-Ig; ■, EL4-gp96-Ig + IL-2S4B6.
Importantly, MST and overall survival, as well as total CD8+ T-cell expansion in response to IL-2S4B6, were independent of transgenic OT-I presence (Figure 5E; supplemental Figure 6). Notably, in vaccinated recipients not receiving OT-I cells, IL-2S4B6 elicited enhanced levels of CD8+ T-effector cells (supplemental Figure 7). To determine if tumor-reactive non-TCR transgenic CD8+ T cells were elicited following gp96-Ig vaccination, DimerX−Kb loaded with peptide was used to detect OVA257-264-specific cells. Importantly, endogenous CD8+ T lymphocytes specific for OVA257-264 were readily apparent in recipients of gp96-Ig vaccination, whereas a markedly increased CD8+ effector T-cell response was only elicited following vaccination together with IL-2S4B6 treatment (Figure 5F; supplemental Figure 7). To test the vaccine strategy using a system without OVA or OT-I T cells, a highly lethal minimal residual lymphoma model using intraperitoneal inoculation of 5000 EL-4 lymphoma cells 1 day after HSCT was used.60,61 The results illustrated that EL4-gp96-Ig vaccination in combination with IL-2S4B6 significantly extended MST compared with monotherapy with either reagent (Figure 5G). Moreover, the MST of the individual treatments were indistinguishable from nonvaccinated mice (Figure 5G).
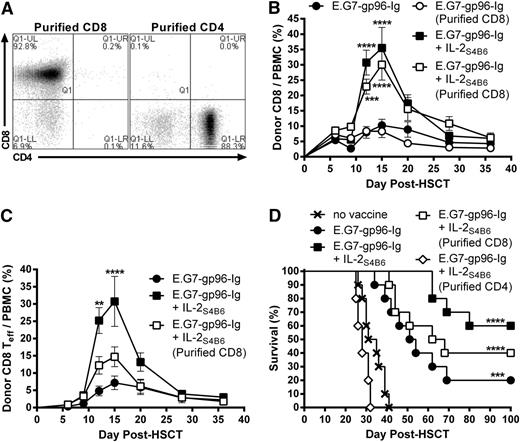
To identify which donor leukocyte populations were required for augmented antitumor immunity following vaccine and IL-2 therapy (Figure 5), donor TCD BM was supplemented with purified CD8+ or CD4+ T cells without addition of OT-I (Figure 6A). Interestingly, donor CD8+ T cells expanded following vaccine and IL-2S4B6 treatment in the absence of donor CD4+ T cells (Figure 6B; supplemental Figure 8). However, a significantly smaller portion of these expanded donor CD8+ T cells transitioned to effector cells (CD62L−CD44+; Figure 6C). These recipients also trended to shortened MST and overall survival compared with recipients of enriched total T cells (Figure 6D). Importantly, recipients of purified CD4+ T cells treated with gp96-Ig vaccination and IL-2S4B6 displayed no survival benefit compared with nonvaccinated recipients transplanted with enriched total T cells (Figure 6D).
Donor non-CD8+ cells were required for optimal antitumor immunity induced by combination therapy with gp96-Ig-secreting tumor cell vaccination and IL-2 cytokine-antibody complexes in syngeneic HSCT recipients with lymphoma. Transplants, tumor inoculation, and treatments were performed as in Figure 5; however, some recipients received BM supplemented with purified CD4+ (≥88%) or CD8+ (≥90%) T cells in the absence of OT-I. (A) CD4+ and CD8+ T-cell content of purified CD4+ or CD8+ T cells. (B) Donor CD8+ T cells efficiently expanded following vaccination and IL-2S4B6 in the absence of donor CD4+ T cells. Donor CD8+ T-cell frequency in the peripheral blood; n = 5; ●, E.G7-gp96-Ig; ○, E.G7-gp96-Ig (purified CD8); ■, E.G7-gp96-Ig + IL-2S4B6; □, E.G7-gp96-Ig + IL-2S4B6 (purified CD8). The results for E.G7-gp96-Ig + IL-2S4B6 and E.G7-gp96-Ig + IL-2S4B6 (purified CD8) were repeated in an independent experiment. (C) In the absence of donor CD4+ T cells, donor CD8+ T-effector cells were not efficiently generated following vaccine and IL-2S4B6. Donor CD8+ Teff (CD62L− CD44+) cell frequency in the peripheral blood; n = 5 from a representative of 2 experiments; ●, E.G7-gp96-Ig; ■, E.G7-gp96-Ig + IL-2S4B6; □, E.G7-gp96-Ig + IL-2S4B6 (purified CD8). The results for E.G7-gp96-Ig + IL-2S4B6 and E.G7-gp96-Ig + IL-2S4B6 (purified CD8) were repeated in an independent experiment. (D) Survival benefit of vaccination and IL-2S4B6 therapy is reduced in lymphoma-bearing HSCT recipients receiving TCD-BM supplemented with purified CD8+ T cells and abolished in recipients of purified CD4+ T cells; n = 5 to 10 from a pool of 3 experiments; x, no vaccine; ●, E.G7-gp96-Ig; ■, E.G7-gp96-Ig + IL-2S4B6; □, E.G7-gp96-Ig + IL-2S4B6 (purified CD8); ◊, E.G7-gp96-Ig + IL-2S4B6 (purified CD4).
Donor non-CD8+ cells were required for optimal antitumor immunity induced by combination therapy with gp96-Ig-secreting tumor cell vaccination and IL-2 cytokine-antibody complexes in syngeneic HSCT recipients with lymphoma. Transplants, tumor inoculation, and treatments were performed as in Figure 5; however, some recipients received BM supplemented with purified CD4+ (≥88%) or CD8+ (≥90%) T cells in the absence of OT-I. (A) CD4+ and CD8+ T-cell content of purified CD4+ or CD8+ T cells. (B) Donor CD8+ T cells efficiently expanded following vaccination and IL-2S4B6 in the absence of donor CD4+ T cells. Donor CD8+ T-cell frequency in the peripheral blood; n = 5; ●, E.G7-gp96-Ig; ○, E.G7-gp96-Ig (purified CD8); ■, E.G7-gp96-Ig + IL-2S4B6; □, E.G7-gp96-Ig + IL-2S4B6 (purified CD8). The results for E.G7-gp96-Ig + IL-2S4B6 and E.G7-gp96-Ig + IL-2S4B6 (purified CD8) were repeated in an independent experiment. (C) In the absence of donor CD4+ T cells, donor CD8+ T-effector cells were not efficiently generated following vaccine and IL-2S4B6. Donor CD8+ Teff (CD62L− CD44+) cell frequency in the peripheral blood; n = 5 from a representative of 2 experiments; ●, E.G7-gp96-Ig; ■, E.G7-gp96-Ig + IL-2S4B6; □, E.G7-gp96-Ig + IL-2S4B6 (purified CD8). The results for E.G7-gp96-Ig + IL-2S4B6 and E.G7-gp96-Ig + IL-2S4B6 (purified CD8) were repeated in an independent experiment. (D) Survival benefit of vaccination and IL-2S4B6 therapy is reduced in lymphoma-bearing HSCT recipients receiving TCD-BM supplemented with purified CD8+ T cells and abolished in recipients of purified CD4+ T cells; n = 5 to 10 from a pool of 3 experiments; x, no vaccine; ●, E.G7-gp96-Ig; ■, E.G7-gp96-Ig + IL-2S4B6; □, E.G7-gp96-Ig + IL-2S4B6 (purified CD8); ◊, E.G7-gp96-Ig + IL-2S4B6 (purified CD4).
To assess the memory response in previously vaccinated HSCT recipients, surviving mice (>100 days after HSCT; Figure 4-6) were challenged with a lethal number (1.0 × 106) of E.G7 lymphoma cells subcutaneously to determine if tumor-specific memory had been generated. Surviving HSCT recipients (n = 20) previously vaccinated with tumor cells secreting gp96-Ig alone (n = 6) or in combination with IL-2S4B6 (n = 14) exhibited a rejection rate of 90% (supplemental Table 1). The animals that were not protected following rechallenge (2/20) were greater than 1 year of age. Untreated, nontransplanted mice were also not protected (9/9; see supplemental Figure 9, left for kinetics of tumor growth and supplemental Figure 9, right for recipient survival). Interestingly, recipients transplanted with purified CD8+ T cells (n = 2) also rejected this subcutaneous tumor challenge (data not shown).
Administration of directed IL-2 enhanced innate and adaptive immunity in the early post-HSCT period
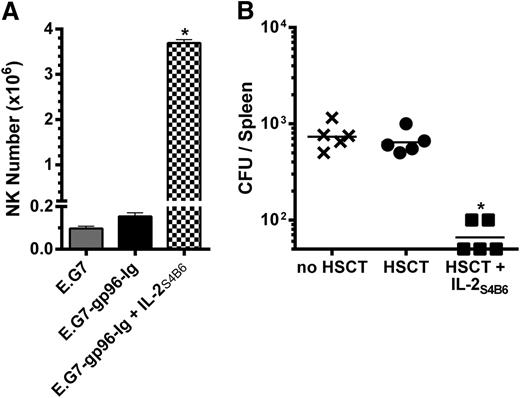
The vaccine site was analyzed following 3 treatments with IL-2S4B6 (during the peak of the CD8+ T-cell response; Figure 5A) to address the effect of directed IL-2 on NK cells after transplant.62 Notably, NK cell numbers were significantly increased at the site of vaccination (>20×) compared with non-IL-2-treated recipients (Figure 7A). To determine if the IL-2-induced increases in NK cells and CD8+ T lymphocytes altered antipathogen immunity early after HSCT, recombinant L monocytogenes was inoculated intravenously into mice 2 weeks after HSCT. One week after inoculation, untreated nontransplanted mice and HSCT recipients contained a similar number of CFUs in the spleen (Figure 7B). IL-2S4B6-treated HSCT recipients exhibited elevated CD8+ T-cell levels following 4 IL-2S4B6 infusions (supplemental Figure 10) and significantly fewer CFU numbers 1 week later (Figure 7B).
IL-2 complex therapy enhanced NK cell numbers and antipathogen immunity in HSCT recipients. (A) IL-2S4B6 therapy elicited large numbers of NK cells in the peritoneal cavity of intraperitoneally vaccinated HSCT recipients 5 days following the third treatment. NK1.1+ cell numbers in the peritoneal cavity; n = 2. (B) IL-2S4B6-treated HSCT recipients displayed fewer splenic bacterial CFU following intravenous inoculation with 1.0 × 104 CFU L monocytogenes 14 days after HSCT and 4 infusions of IL-2S4B6 starting 3 days after HSCT and repeated every 3 days. CFU numbers in the spleen 7 days after infection; n = 5 from representative of 2 experiments; x, no HSCT; ●, HSCT; ■, HSCT + IL-2S4B6.
IL-2 complex therapy enhanced NK cell numbers and antipathogen immunity in HSCT recipients. (A) IL-2S4B6 therapy elicited large numbers of NK cells in the peritoneal cavity of intraperitoneally vaccinated HSCT recipients 5 days following the third treatment. NK1.1+ cell numbers in the peritoneal cavity; n = 2. (B) IL-2S4B6-treated HSCT recipients displayed fewer splenic bacterial CFU following intravenous inoculation with 1.0 × 104 CFU L monocytogenes 14 days after HSCT and 4 infusions of IL-2S4B6 starting 3 days after HSCT and repeated every 3 days. CFU numbers in the spleen 7 days after infection; n = 5 from representative of 2 experiments; x, no HSCT; ●, HSCT; ■, HSCT + IL-2S4B6.
Discussion
Overcoming diminished immune function is a major challenge following regimens involving aggressive therapy and autologous HSCT. An ideal strategy would simultaneously elicit rapid and potent tumor-specific responses while successfully augmenting overall antipathogen immunity. The principal objective of this study was to evaluate the efficacy of a tumor cell vaccine combined with IL-2 in experimental models of minimal residual lymphoma during the early period following myeloablative conditioning and syngeneic HSCT. The results demonstrated that vaccination with tumor cells engineered to secrete gp96-Ig initiated within a few days after HSCT induced the activation, expansion, and functional competence of tumor-reactive CD8+ T lymphocytes. Notably, administration of IL-2 prebound to an anti-IL-2 mAb led to rapid and extensive expansion of total CD8+ T lymphocytes and NK cells and supported the generation of antipathogen responses. Overall, this strategy of coupling directed IL-2 to heat shock protein vaccination induced remarkable expansion of effector CD8+ T lymphocytes and led to dramatic increases in MST and overall survival in HSCT recipients, as well as the generation of long-lived antitumor memory.
Clinical studies have demonstrated that tumor-specific CD8+ T-cell responses can be elicited in myeloma patients within 1 to 2 months after autologous HSCT.63 Responses in these patients were generated using multiepitope predicted tumor antigen vaccines against human telomerase reverse transcriptase and survivin. Following reinfusion of autologous T cells 2 days after HSCT and multiple vaccinations beginning at 2 weeks, approximately one-third of patients receiving a prime-boost regimen exhibited specific responses to the predicted tumor antigens. Similarly, we sought to transfer T cells from tumor-bearing donors and used a multiepitope vaccine in the early post-HSCT period to induce robust and specific immunity.64 Our strategy differed as it did not use ex vivo stimulation, but included IL-2 together with a tumor vaccine in vivo to induce massive CD8+ T-cell expansion early after HSCT. In contrast to clinical studies in which patients apparently required vaccination prior to T-cell collection and HSCT to elicit responses to vaccine antigens,65,66 the inoculum used here was obtained from donor mice bearing tumors but not previously vaccinated. The present study demonstrated that vaccination was absolutely required to elicit expansion of an endogenous antigen-specific T-cell clone (Figure 5F). Nonetheless, the response of this individual non-TCR transgenic tumor-reactive CD8+ T-cell population cannot account for the markedly increased survival observed in recipients of the combinatorial strategy described here. We hypothesize that additional tumor-specific clones, which cannot be directly monitored, resulting from the multiepitope nature of the heat shock protein vaccine, must contribute to the increased survival observed in recipients of vaccine and IL-2. Based on the enormous increase in CD8+ T-effector cell numbers following combination treatment (Figure 5F), we speculate this response includes low TCR affinity clones that require strong stimulating signals and can be expanded in the presence of IL-2S4B6.
There have been few preclinical reports concerning therapeutic antitumor vaccination in myeloablative models of syngeneic HSCT supplemented with T cells. In contrast to the multipronged vaccination strategy used here, those studies required tumor cell vaccination after de novo T-cell genesis following transplant, higher doses of donor T cells (>2.0 × 106), or presensitization of donor T cells with the tumor vaccine.67-69 One objective of our study was to rapidly elicit T-cell responses by transplanted donor cells prior to de novo production of thymic-derived T cells. Expansion of donor tumor-reactive CD8+, but not CD4+, T cells was observed following gp96-Ig vaccination alone or together with directed IL-2 within 10 days (Figure 4B; supplemental Figure 3). However, only this combination strategy induced a marked increase in overall CD8+ T effector cell numbers (∼500%) ≤2 weeks after HSCT (Figure 5F). This time frame is prior to production and emigration of new thymic-derived T cells, which takes place 2 to 3 weeks after HSCT.70 Together with the observation that gp96-Ig vaccination alone or together with IL-2S4B6 did not evoke protective immunity in recipients receiving TCD BM grafts (Figure 4C) or purified CD4+ T cells (Figure 6D), respectively, we conclude that the vaccination regimens here expanded transplanted donor CD8+ T cells very early following HSCT, paralleling clinical findings requiring the addition of large numbers of T cells to provide detectable immunity.65
Few preclinical studies have incorporated heat shock protein preparations into vaccine strategies for treating hematologic malignancies, including myeloma.71,72 In the context of experimental syngeneic HSCT, multiple vaccinations with purified autologous lymphoma-derived gp96 resulted in increased survival in a model of minimal residual lymphoma.73-75 In contrast to the present studies, 2 to 4 times the amount of gp96 was required, and the first vaccination was administered 2 weeks after HSCT, with subsequent vaccinations after the emergence of thymic-derived T cells. Notably, in our immediate vaccination strategy, although recipient APCs initially contributed to eliciting tumor-reactive CD8+ T cells (Figure 2A), the response rapidly became singularly dependent on donor B7-expressing APCs (Figure 2B), which included a critical CD11c+ CD8α+ cross-presenting DC population (Figure 2C) and required perforin-1 (Figure 2D). Therefore, enriched donor T cells plus CD34+ progenitors may not be optimal for use with this vaccine strategy. The role of perforin-1 remains obscure, but could involve NK-APC interactions early during the response.76-78
The initial use of IL-2 in combination with vaccination or complexed to an anti-IL-2 mAb in the 1990s reported increased CD8+ T-cell proliferation and enhanced antitumor immunity in vivo.79,80 The transplant system used here was uniquely suitable for IL-2S4B6 complex use because virtually all CD8+ T cells transplanted into lymphopenic hosts assume a central-memory phenotype (CD62L+ CD44+ CD122+ CD127+ KLRG-1−; data not shown), expressing IL-2 receptor β and γ chains presumably rendering these donor CD8+ T cells sensitive to IL-2S4B6 stimulation.55,56 Boyman et al demonstrated rapid CD8+ T-cell expansion following daily (1 week) administration of IL-2S4B6 after HSCT.33 We detected virtually no change in CD4+ T-cell and Treg levels (supplemental Figure 2), indicating that under such conditions, IL-2S4B6 complex most efficiently targeted transplanted donor CD8+ T cells. Notably, our data illustrated that vaccination increased the frequency and numbers of tumor-reactive CD8+ T cells and subsequently IL-2S4B6 markedly amplified CD8+ effector T-cell levels, which directly correlated with the observed increases in MST and overall survival. Furthermore, in an independent, highly lethal model of minimal residual lymphoma, increased MST was only observed following vaccine and IL-2 (Figure 5G). Studies have demonstrated that human IL-2 complexed to human anti-IL-2 mAb effectively expanded murine CD8+ T cells and enhanced antitumor immunity.33-35 Interestingly, Levin et al engineered human IL-2 to possess binding properties similar to those when natural IL-2 is complexed to anti-IL-2 mAb.81 These human IL-2 complexes, “super-IL-2,” and new IL-2 fusion products may therefore provide next-generation reagents to implement and clinically test the regimen developed here.
The individual capacities of gp96 and IL-2 to activate APCs and NK cells, respectively, is well documented,17,18,62,82 and therefore CD4+ T-cell presence may not be required for post-HSCT vaccination. IL-2S4B6 treatment following transplant with purified CD8+ T cells (Figure 6A; supplemental Figure 8) resulted in marked donor CD8+ T-cell expansion (Figure 6B). However, their effector cell phenotype (CD62L−CD44+) was diminished (Figure 6C), which might have contributed to reduced survival in these recipients (Figure 6D). These findings support the notion that donor CD4+ T-cell licensing of APCs is unnecessary for this CD8+ T-cell expansion, and purified donor CD4+ T cells failed to provide detectable antitumor immunity following vaccine and IL-2. Furthermore, we observed potent NK cell expansion following vaccination and IL-2S4B6 infusion (Figure 7A), a population critical for gp96-Ig-based strategies.30 Thus, we posit IL-2S4B6 enhanced vaccine efficacy in both an indirect and direct fashion via NK cell and tumor-reactive CD8+ T-lymphocyte expansion. Experiments are currently underway to elucidate the role of NK cells, and initial observations indicated that depletion of NK cells in HSCT recipients abolished vaccine and IL-2 effectiveness (data not shown). In addition to relapse of primary disease, infection is the second leading cause of mortality in HSCT recipients.1 Both CD8+ T lymphocytes and NK cells contribute to immunity against L monocytogenes.47,48,83-86 IL-2S4B6 treatment following HSCT resulted in diminished splenic bacterial levels (Figure 7B). This is the first demonstration we are aware of reporting IL-2 enhanced antipathogen immunity early following myeloablative conditioning and syngeneic HSCT.
Srivastava and colleagues introduced autologous tumor-derived gp96 preparations into patients with cancer, including lymphoma.87,88 Although immune correlates were elicited, overall patient survival was relatively unchanged compared with standard of care regimens.89 Notably, the first successful phase 1 trial using a human allogeneic lung cancer cell line engineered to secrete gp96-Ig was recently completed.29 Interestingly, ∼75% of evaluable patients displayed CD8+ IFN-γ responses against the vaccine cells, and these subjects had an MST of 16.5 vs 4.5 months for nonresponders. The present findings support the notion that a more effective combinatorial strategy can first activate and then expand tumor-reactive CD8+ T cells. The tumor cell secreted heat shock protein vaccine and directed IL-2 regimen developed in the preclinical model here represents a promising strategy with translational applications for patients with hematologic cancers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Henry Barreras for technical assistance and Eli Gilboa for reading the manuscript and providing helpful comments. The authors appreciated the help of Tomomi Toubai and Katherine Oravecz-Wilson in the laboratory of P. Reddy for providing Batf3-deficient bone marrow cells. The authors thank the Sylvester Comprehensive Cancer Center Flow Cytometry Facility.
The authors acknowledge the support of the Sylvester Comprehensive Cancer Center and the Sheila and David Fuente Graduate Program in Cancer Biology. This work was supported by National Institutes of Health, Cancer Institute and Institute of Allergy and Infectious Disease grants R01CA120776 and R01AI046689 (R.B.L.), P01CA109094 and P01AI096396 (E.R.P.), and R01AI055815 (T.R.M.).
Authorship
Contribution: R.G.N. performed experiments and collected data; M.J.D. assisted in performing experiments and collecting data involving L monocytogenes studies; R.G.N. and R.B.L. designed research and analyzed and interpreted data; M.J.D. and T.R.M. assisted in designing research and analyzing and interpreting data involving L monocytogenes studies; R.G.N. performed statistical analysis; R.G.N. and R.B.L. made the figures and wrote the manuscript; and T.R.M. and E.R.P. read and critiqued the manuscript and contributed reagents.
Conflict-of-interest disclosure: E.R.P. and the University of Miami have a financial interest in the commercial development of gp96-Ig-based vaccines. The remaining authors declare no competing financial interests.
Correspondence: Robert B. Levy, Department of Microbiology and Immunology, University of Miami Miller School of Medicine, 1600 NW 10th Ave, Miami, FL 33101; e-mail: rlevy@med.miami.edu.