To the editor:
Brentuximab vedotin (BV) is a novel anti-CD30 antibody drug conjugate that gained accelerated regulatory approval1 after studies demonstrating impressive efficacy in relapsed Hodgkin lymphoma and anaplastic large cell lymphoma.2-4 The cytotoxic component is monomethylauristatin E, a potent inhibitor of microtubule assembly. Although abdominal pain has been reported in up to 18% of patients treated with BV, pancreatitis is a previously unidentified serious adverse event (while this report was under review, an additional case of BV-associated pancreatitis was reported in which the patient was successfully retreated5 ). After a fatal case of pancreatitis in a patient receiving single-agent BV in an ongoing clinical trial (NCT01476410), we identified an additional fatal case and 6 nonfatal cases of pancreatitis associated with single-agent BV therapy. Cases were collected from collaborating investigators and colleagues at other lymphoma programs, including adverse events reported to the US Food and Drug Administration Adverse Event Report System from June 2011 to July 2013. Institutional review board or ethics committee approval, as required, was obtained in all cases. Collected data were centralized through the Research on Adverse Drug Events and Reports project at Northwestern University in Evanston, Illinois. Immunohistochemical staining with the anti-CD30 antibody BER-H2 (Dako) was performed on residual normal pancreas from 1 of the fatal cases and on 2 normal control pancreas specimens and was analyzed by routine microscopy and the Nuance multispectral imaging system (PerkinElmer).6
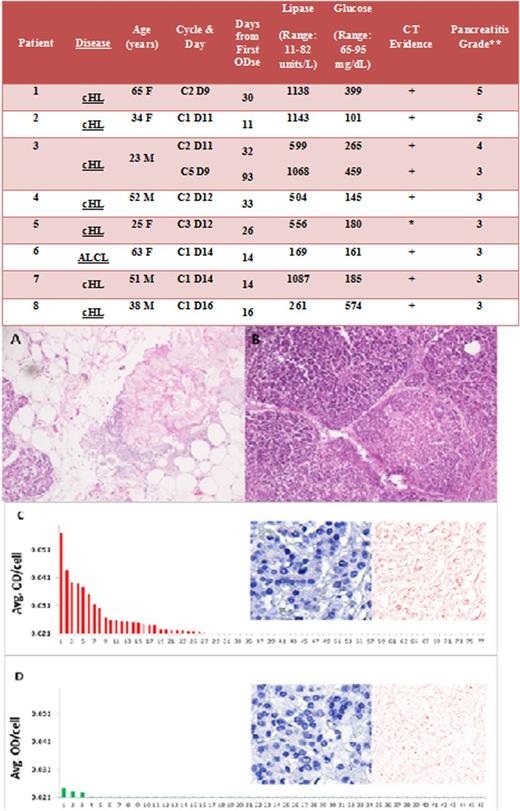
Demographic, treatment, and laboratory data for all 8 cases are summarized here (Figure 1). Notably, no patient had antecedent exposure to alcohol, had biliary pathology diagnosed during their ensuing hospital course, or had a history of hypertriglyceridemia. The median time to presentation was 26 days from the first BV exposure and 12 days from the most recent treatment, with all cases occurring by the third cycle of therapy. All patients had clinical presentations consistent with pancreatitis, as manifested by severe abdominal pain and nausea along with biochemical evidence, with an elevated lipase. Two patients were retreated with BV after their initial episodes of pancreatitis, including one patient who developed recurrent pancreatitis and one who never redeveloped symptoms. Two patients experienced progressive multiorgan dysfunction as a consequence of pancreatitis, leading to death.
Clinical characteristics of patients with pancreatitis after treatment with BV, and autopsy specimen histology and quantitative immunohistochemistry with multispectral imaging (patient 1). (A) Autopsy specimen demonstrating fat necrosis of pancreas. (B) Autopsy specimen demonstrating coagulation necrosis of pancreas. (C) CD30 immunohistochemistry slide. Histogram: CD30 positivity in 27 (35%) of the 77 analyzed cells, with background optical density (OD) subtracted from all values. The bars signify specific CD30 staining. Insert: Standard light microscopic images of CD30 immunohistochemistry slide (left) and corresponding computer-generated image of pure 3,3′-diaminobenzidine signal (right), signifying CD30 expression in this biopsy. (D) Mouse immunoglobulin control slide. Histogram: The average OD values per cell are depicted. Here, a small subset of cells show OD values that exceed the range of 2 standard deviations from mean, an expected finding assuming a normal distribution of OD values. Insert: Standard light microscopic image of mouse immunoglobulin slide (left) and the corresponding computer-generated image of pure 3,3′-diaminobenzidine signal (right), showing nonspecific staining. Note: Data are expressed as average OD per cell. CD30 positivity is defined as any average OD per cell greater than nonspecific background OD (in this case, 0.021 plus 2 standard deviations from the mean, a value determined by measuring OD values in the mouse immunoglobulin control slide). cHL, classical Hodgkin Lymphoma; ALCL, anaplastic large cell lymphoma; CT, computed tomography. *Ultrasound showed only biliary sludge; **CTCAE 4.03 (evs.nci.nih.gov).
Clinical characteristics of patients with pancreatitis after treatment with BV, and autopsy specimen histology and quantitative immunohistochemistry with multispectral imaging (patient 1). (A) Autopsy specimen demonstrating fat necrosis of pancreas. (B) Autopsy specimen demonstrating coagulation necrosis of pancreas. (C) CD30 immunohistochemistry slide. Histogram: CD30 positivity in 27 (35%) of the 77 analyzed cells, with background optical density (OD) subtracted from all values. The bars signify specific CD30 staining. Insert: Standard light microscopic images of CD30 immunohistochemistry slide (left) and corresponding computer-generated image of pure 3,3′-diaminobenzidine signal (right), signifying CD30 expression in this biopsy. (D) Mouse immunoglobulin control slide. Histogram: The average OD values per cell are depicted. Here, a small subset of cells show OD values that exceed the range of 2 standard deviations from mean, an expected finding assuming a normal distribution of OD values. Insert: Standard light microscopic image of mouse immunoglobulin slide (left) and the corresponding computer-generated image of pure 3,3′-diaminobenzidine signal (right), showing nonspecific staining. Note: Data are expressed as average OD per cell. CD30 positivity is defined as any average OD per cell greater than nonspecific background OD (in this case, 0.021 plus 2 standard deviations from the mean, a value determined by measuring OD values in the mouse immunoglobulin control slide). cHL, classical Hodgkin Lymphoma; ALCL, anaplastic large cell lymphoma; CT, computed tomography. *Ultrasound showed only biliary sludge; **CTCAE 4.03 (evs.nci.nih.gov).
On pathologic assessment, neither our patient’s autopsy pancreas specimen nor normal pancreas controls showed CD30-positivity with routine immunohistochemistry. However, using multispectral imaging, the patient’s routine immunohistochemistry pancreas sample and 2 normal pancreas controls showed variable low-level CD30 expression that was significantly higher (P < .0001, Student t test) than mouse immunoglobulin controls (Figure 1), suggesting that unintended targeting of low-level pancreatic CD30 by BV may underlie this complication of therapy. Other mechanisms postulated for the observed toxicity include circulating unconjugated monomethylauristatin E, the presence of isolated CD30-positive malignant cells in the pancreas of some patients, or host factors such as mutations predisposing patients to the development of acute or chronic pancreatitis.7
BV offers a promising therapeutic advance for the treatment of CD30-positive lymphomas, with limited toxicity. This series demonstrates an uncommon, previously unreported, and potentially life-threatening adverse event. Pancreatitis should be considered in the differential diagnosis of abdominal pain after BV therapy.
Authorship
Acknowledgment: We gratefully acknowledge the statistical assistance of Victoria P. Rajmanickam with regard to the multispectral imaging data.
This work was supported by the following grants: Leukemia Lymphoma Society, Special fellow in Clinical Research, 4587-11 (N.G.); National Institutes of Health, National Cancer Institute 5R01 CA 102713-04 and 5R01 CA 125077-03 (D.P.W.); Department of Veterans Affairs (G.S.W.); National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant T32AR055893 (K.S.).
Contribution: M.D.G. and J.N.W. designed the study, collected and analyzed the data, and wrote the manuscript; A.M.E., T.S.F., P.H., B.C., A.E., A.J.M., N.G., A.M.P., S.M.T., and L.I.G. assisted in collecting cases, reviewed the data, and assisted in writing the manuscript; J.L. and A.C. analyzed the autopsy and provided the pathology figures; B.N., D.W.R., and D.P.W. collected and analyzed the data, reviewed the manuscript, and provided an analysis of FAERS; and K.S. and G.S.W. performed, analyzed, and interpreted the quantitative multispectral immunohistochemistry with the Nuance system.
Conflict-of-interest disclosure: A.M.E., A.J.M., A.M.P., L.I.G., and J.N.W. received research funding from Seattle Genetics; T.S.F. is a consultant to Seattle Genetics; P.H. is a consultant to and received honoraria from Seattle Genetics; B.C. and N.G. are on the advisory committee for Millennium Pharmaceuticals; and A.E. is a consultant to Takeda, Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jane N. Winter, Northwestern University Feinberg School of Medicine, Division of Hematology/Oncology, 676 North Saint Clair St, Suite 850, Chicago, IL 60611; e-mail: j-winter@northwestern.edu.