Abstract
Despite their small size and anucleate status, platelets have diverse roles in vascular biology. Not only are platelets the cellular mediator of thrombosis, but platelets are also immune cells that initiate and accelerate many vascular inflammatory conditions. Platelets are linked to the pathogenesis of inflammatory diseases such as atherosclerosis, malaria infection, transplant rejection, and rheumatoid arthritis. In some contexts, platelet immune functions are protective, whereas in others platelets contribute to adverse inflammatory outcomes. In this review, we will discuss platelet and platelet-derived mediator interactions with the innate and acquired arms of the immune system and platelet-vessel wall interactions that drive inflammatory disease. There have been many recent publications indicating both important protective and adverse roles for platelets in infectious disease. Because of this new accumulating data, and the fact that infectious disease continues to be a leading cause of death globally, we will also focus on new and emerging concepts related to platelet immune and inflammatory functions in the context of infectious disease.
Introduction
Platelets are best known as the cellular mediator of thrombosis. There is now a growing appreciation of the important immune and inflammatory roles of platelets in both health and disease. A number of studies have demonstrated that platelets impact inflammatory processes ranging from atherosclerosis to infectious diseases, making platelets the most numerous circulating cell type that has an immune function. Platelets interact with white blood cells and vascular endothelial cells both directly by contact-dependent mechanisms and indirectly through secreted immune mediator-driven mechanisms. Platelet immune effects are therefore noted both locally at sites of platelet activation and deposition or systemically at locations distant from platelet activation itself.1,2 Platelet interactions with inflammatory cells may mediate proinflammatory outcomes, but these interactions have likely evolved to be beneficial in limiting infection. For example, with a breach in the skin there is exposure to pathogens, and by combining thrombotic and immune recruitment functions, platelets may help focus hemostasis and immune responses against potential infectious agents to prevent pathogen invasion. However, continued or chronic platelet interactions with white blood cells or endothelial cells can lead to adverse effects from excessive immune stimulation and inflammatory insult.
Platelets are small in size (∼4 µm), but because they are present in large numbers (≈200 000/µL blood in humans) and have many preformed inflammatory molecules and immune mediators, platelets exert inflammatory effects on a scale that exceeds their individual size. A platelet contains ∼60 granules that store many molecules with immune functions (Table 1, 2-59 partial list of granule contents with inflammatory/immune roles).60 There are 3 types of platelet granules: α-granules, dense granules, and lysomal granules. A recent report has described a possible new type of granule termed a T-granule.61 α-Granules are the most numerous (50-60 per platelet) and largest (200-400 nm) platelet granule and store a large variety of proteins. One proteomic analysis of α-granule content found 284 proteins.62 Dense granules are smaller (∼150 nm), less numerous (3-8 per platelet), and store small molecules (Table 1). Lysosomal granules are sparse and contain glycohydrolases and degradative enzymes.63-66 Upon platelet stimulation, granules undergo regulated exocytosis and release their contents into the extracellular environment, or molecules found on the inner granule membrane become surface expressed. Although granule exocytosis contributes to platelet activation and thrombus formation, many granule-derived mediators also have either primary or secondary roles as immune molecules.
Partial list of platelet-derived inflammatory mediators and immune modulators
| Molecule . | Immune/inflammatory role . |
|---|---|
| α-Granule | |
| PF4 | Chemokine: monocyte, neutrophil, and T-cell recruitment; Th differentiation2-5 |
| Ppbp β-thromboglobulin NAP-2 | Chemokine: neutrophil activation and recruitment, macrophage phagocytic activity6-8 |
| P-selectin | Selectin: leukocyte adhesion, complement activation9-11 |
| CD40L | TNF superfamily: antigen-presenting cell activation, B-cell responses, endothelial cell activation12-14 |
| TGF-β | Cytokine: cell proliferation, T-cell differentiation, B-cell and macrophage phenotype regulation15-19 |
| PDGF | Growth factor: cell growth and differentiation, monocyte/macrophage differentiation20,21 |
| VWF | Platelet adhesion, PMN extravasation22,23 |
| CD63 | Tetraspanin: transmembrane adaptor protein, leukocyte recruitment24 |
| SDF-1 | Chemokine: T-cell, monocyte, and PMN chemotaxis25-27 |
| VEGF | Growth factor: angiogenesis, adhesion molecule expression28-30 |
| Thrombospondins | Apoptosis, endothelial cell inflammation, macrophage-platelet aggregates31,32 |
| MIP-1α | Cytokine: neutrophil and eosinophil activation, B-cell immunoglobulin production33,34 |
| MMP-2, MMP-9 | Protease: extracellular matrix breakdown, platelet-leukocyte aggregate formation35-38 |
| Cyclophilin A | Vascular smooth muscle cell growth factor36 |
| Dense granule | |
| Serotonin | DC and T-cell functions39,40 |
| Glutamate | T-cell trafficking41,42 |
| Polyphosphates | Inflammatory response amplification43,44 |
| ADP | Platelet, leukocyte, endothelial cell activation45-47 |
| Histamine | Increased vessel reactivity and degranulation48-50 |
| Produced or constitutively expressed | |
| IL-1β | Cytokine: acute phase response, leukocyte and endothelial activation51-54 |
| Thromboxane | Eicosanoid: T-cell differentiation, monocyte activation55-57 |
| Nitric oxide | Reactive oxygen species: anti-inflammatory and antithrombotic58 |
| GPIbα | Adhesion molecule: binds Mac-1 on leukocytes59 |
| Molecule . | Immune/inflammatory role . |
|---|---|
| α-Granule | |
| PF4 | Chemokine: monocyte, neutrophil, and T-cell recruitment; Th differentiation2-5 |
| Ppbp β-thromboglobulin NAP-2 | Chemokine: neutrophil activation and recruitment, macrophage phagocytic activity6-8 |
| P-selectin | Selectin: leukocyte adhesion, complement activation9-11 |
| CD40L | TNF superfamily: antigen-presenting cell activation, B-cell responses, endothelial cell activation12-14 |
| TGF-β | Cytokine: cell proliferation, T-cell differentiation, B-cell and macrophage phenotype regulation15-19 |
| PDGF | Growth factor: cell growth and differentiation, monocyte/macrophage differentiation20,21 |
| VWF | Platelet adhesion, PMN extravasation22,23 |
| CD63 | Tetraspanin: transmembrane adaptor protein, leukocyte recruitment24 |
| SDF-1 | Chemokine: T-cell, monocyte, and PMN chemotaxis25-27 |
| VEGF | Growth factor: angiogenesis, adhesion molecule expression28-30 |
| Thrombospondins | Apoptosis, endothelial cell inflammation, macrophage-platelet aggregates31,32 |
| MIP-1α | Cytokine: neutrophil and eosinophil activation, B-cell immunoglobulin production33,34 |
| MMP-2, MMP-9 | Protease: extracellular matrix breakdown, platelet-leukocyte aggregate formation35-38 |
| Cyclophilin A | Vascular smooth muscle cell growth factor36 |
| Dense granule | |
| Serotonin | DC and T-cell functions39,40 |
| Glutamate | T-cell trafficking41,42 |
| Polyphosphates | Inflammatory response amplification43,44 |
| ADP | Platelet, leukocyte, endothelial cell activation45-47 |
| Histamine | Increased vessel reactivity and degranulation48-50 |
| Produced or constitutively expressed | |
| IL-1β | Cytokine: acute phase response, leukocyte and endothelial activation51-54 |
| Thromboxane | Eicosanoid: T-cell differentiation, monocyte activation55-57 |
| Nitric oxide | Reactive oxygen species: anti-inflammatory and antithrombotic58 |
| GPIbα | Adhesion molecule: binds Mac-1 on leukocytes59 |
ADP, adenosine 5′-diphosphate; CD40L, CD40 ligand; DC, dendritic cell; GPIbα, glycoprotein Ibα; IL, interleukin; MIP, macrophage-inflammatory protein; MMP, metalloproteinase; NAP, neutrophil-activating peptide; PDGF, platelet-derived growth factor; PF4, platelet factor 4; PMN, neutrophil; ppbp, proplatelet basic protein; SDF, stromal cell–derived factor; TGF, transforming growth factor; Th, T helper; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; VWF, von Willebrand factor.
α-Granule constituents such as PF4, regulated on activation normal T expressed and secreted (RANTES), SDF-1, and ppbp have limited thrombotic functions and instead are chemokines and cytokines that recruit and activate other immune cells or induce endothelial cell inflammation. The inflammatory roles of most α-granule–derived chemokines, cytokines, and adhesion molecules are well described.67,68 Dense granule constituents such as ADP, serotonin, polyphosphates, and glutamate are more understood as modifiers of platelet activation and thrombus formation, but many have immune cell–modifying effects. DCs express the P2Y12 ADP receptor, and DC P2Y12 activation increases antigen endocytosis and processing.69 Adenosine triphosphate signaling through T-cell P2X7 increases differentiation of CD4+ Th cells toward a proinflammatory Th17 cell type.70 Polyphosphates induce nuclear factor κB activation and the expression of endothelial adhesion molecules,44 and polyphosphates also amplify high-mobility group protein B1–mediated inflammatory signaling through interactions with the receptor for advanced glycosylation endproducts and P2Y1 receptors.43 Platelet dense granules contain glutamate, and glutamate in the periphery can induce T-cell migration.71,72 Platelets are the peripheral source of serotonin, and serotonin increases monocyte differentiation into DCs73 and early naïve T-cell activation.40 Although these dense granule–derived immune modifiers are highly enriched in platelets and have immune functions, the direct contribution of platelet dense granule constituents to immune responses in a disease context is still largely unexplored.
The number of potential interactions, both direct and indirect, between platelets and other cells is extensive, and as a result, a multitude of inflammatory effects can be exerted by platelets both in the local environment and systemically.
Platelets and innate immune responses
The acute phase response (APR) is the earliest response to infection or vascular injury. The APR is typified by the production of acute phase proteins such as C-reactive protein, serum amyloids A and P, complement proteins, and fibrinogen by the liver. Acute phase proteins destroy or inhibit the growth of microbes and exert procoagulant effects that may limit infection by trapping pathogens within local blood clots. Our studies have indicated that platelets induce the APR.51 Following vascular compromise, it may be advantageous to activate the APR simultaneous with platelet activation to localize and limit systemic invasion of potential microbes. interleukin-1β (IL-1β), IL-6, and IL-8 are all potent inducers of the APR. Platelets are not a direct source of IL-6 or IL-8 but are a major source of IL-1β.51 Because IL-1β is not granule stored and is produced upon platelet stimulation, it was surprising to discover IL-1β in platelets, and more unexpected to find pre–messenger RNA (mRNA) for IL-1β expressed by platelets.53 With platelet stimulation, IL-1β pre-mRNA is spliced, IL-1β mRNA translated into pro-IL-1β, and caspase-1 processed, resulting in the release of functional IL-1β.53 Typical markers of platelet activation (granule exocytosis and integrin activation) are increased rapidly after platelet stimulation (seconds to minutes), but the release of IL-1β from stimulated platelets occurs over hours.74 Platelets contain other mRNAs and pre-mRNAs, some of which are used to make proteins after platelet stimulation, but IL-1β is the best described.75 In a mouse model of severe malaria, we found that platelets are activated early postinfection, and platelet-derived IL-1β has a major role in inducing the APR.51 Platelets were also found localized to hepatic sinusoids following Plasmodium infection indicating that platelets may induce the APR in a contact-dependent manner (Figures 1–2).
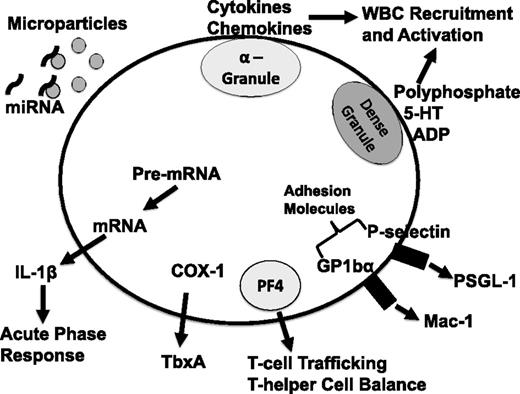
Schematic of platelet-derived immune mediators. 5-HT, serotonin; ADP, adenosine 5′-diphosphate; COX-1, cyclooxygenase 1; P-selectin glycoprotein ligand-1; TbxA, thromboxane; WBC, white blood cell; PSGL-1.
Schematic of platelet-derived immune mediators. 5-HT, serotonin; ADP, adenosine 5′-diphosphate; COX-1, cyclooxygenase 1; P-selectin glycoprotein ligand-1; TbxA, thromboxane; WBC, white blood cell; PSGL-1.
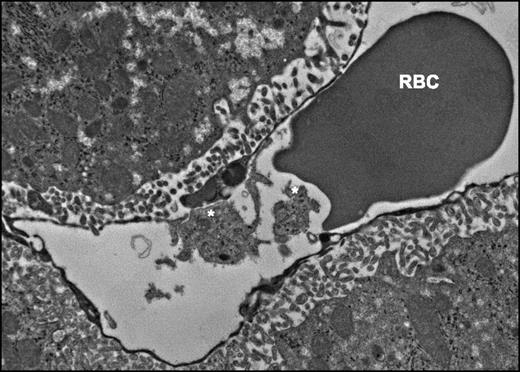
Platelet and liver sinusoid interactions in a Plasmodium berghei–infected mouse (* denotes platelets).
Platelet and liver sinusoid interactions in a Plasmodium berghei–infected mouse (* denotes platelets).
Toll-like receptors (TLRs) are a highly conserved family of pattern recognition receptors expressed by organisms from flies to mammals. TLRs bind pathogen-associated molecular pattern molecules that are broadly expressed by many infectious organisms. Lipopolysaccharide (LPS) is the most studied TLR ligand, and others include unmethylated cytosine guanine dinucleotide, double-stranded RNA, and lipoproteins. Platelets express numerous TLR family members including TLR1, TLR2, TLR4, TLR6, TLR8, and TLR9.76 Because gram-negative sepsis is a major clinical problem with few therapeutic options, much focus has been on the implications and mechanisms of LPS signaling via platelet TLR4 in sepsis-associated thrombocytopenia and platelet activation and accumulation in organs such as the lung.77 CD14 is a coreceptor for LPS signaling through TLR4, but CD14 itself may not be expressed by platelets,78 meaning platelets are dependent on other sources of soluble CD14 to respond to LPS. Platelet TLR4 signaling leads to platelet activation, the shedding of IL-1β-rich microparticles (MPs), and platelet interactions with other cells.74 TLR4-stimulated platelet interactions with adherent neutrophils lead to the formation of neutrophil extracellular traps that may trap bacteria.79 TLR2 also has described roles in platelet inflammatory functions. Stimulation of platelet TLR2 by Porphyromonas gingivalis results in increased platelet-neutrophil adhesion,80 and stimulation of megakaryocytes through TLR2 results in the production of platelets with an increased proinflammatory gene and protein expression.81 Other TLRs, pattern recognition receptors, and scavenger receptors also have functions in platelet activation and thrombosis, including TLR9 and CD36, but their role in inflammation and platelet immune cell interactions remains to be determined.82-84
Monocytes and neutrophils are the most numerous innate immune cells in the blood. Monocytes have major roles in chronic cardiovascular diseases including atherosclerosis. Some of the earliest studies of monocyte interactions with platelets focused on platelet phagocytosis by monocytes within thrombi and speculated that this may contribute to atherosclerotic lesion development.85 The identification of interactions between platelets and monocytes via platelet P-selectin has historical significance, in part because P-selectin is a mediator of platelet and monocyte interactions, and in part because the recognition of this interaction helped accelerate discovery of other platelet immune functions. P-selectin was once alternatively termed platelet activation-dependent granule-external membrane protein or granule membrane protein 140. In 1985, granule membrane protein 140 was shown to be expressed on the platelet plasma membrane after activation,86 setting the stage for other studies demonstrating that P-selectin mediates platelet and leukocyte interactions. This included the in vivo demonstration in a primate arteriovenous shunt model that adherent platelets express P-selectin and platelet P-selectin immobilizes leukocytes at the site of the lesion,10,87 establishing an important framework for subsequent in vivo and disease-focused studies. The generation of the first P-selectin knockout mouse provided genetic proof of a mechanistic role for platelet interactions with leukocytes and the vessel wall.11 The interaction of platelet P-selectin with its receptor on monocytes, PSGL-1, does more than just localize monocytes. P-selectin and PSGL-1 interactions induce tissue factor–bearing MP formation and monocyte proinflammatory changes.88
With the identification of critical links between platelets and monocytes and insights into the association between monocytes and atherosclerosis, an expansion of our understanding of platelets as immune mediators in vascular inflammatory diseases has rapidly emerged. The increase in numbers of commercially available genetically modified mice has also facilitated studies focused on platelet and monocyte interactions in atherosclerosis, an inflammatory disease driven in large part by immune cells.89,90 Early investigations into the pathogenesis of atherosclerosis suggested that platelets induced an endothelial cell inflammatory response91,92 and platelet-derived mediators increased endothelial permeability permitting lipid entry into the vessel wall and atherosclerosis development.93,94 Mouse models have provided evidence that platelet-secreted inflammatory molecules such as RANTES and PF4 localize inflammatory molecules to sites of vascular inflammation, accelerating lesion development.95-97 Human studies have identified increased platelet and monocyte aggregates in the circulation of those with atherosclerosis, perhaps accelerating the immune response to vascular lesions and atherosclerotic progression.98-101 Because of the great health impact of atherosclerosis and the role of innate immune cells in its pathogenesis, studies are likely to continue to define the role of platelets in innate immunity.
Platelets and acquired immune responses
Although the role of platelets in innate immune responses has received much more attention, platelets also influence acquired immune responses, including T-cell trafficking, activation, and differentiation. T cells are broadly divided into CD8+ or CD4+ cells, and CD4+ T cells further divided into the Th types Th1, Th2, or Th17 as immune effectors and T regulatory cells as immune suppressors. CD8+ T cells are often termed cytotoxic T cells. They recognize antigen presented in major histocompatibility complex class I leading to infected cell death through CD8+ T-cell cytokine secretion. CD4+ cells are master regulators of immune responses. CD4+ cells recognize antigen presented by major histocompatibility complex class II and release cytokines that regulate the activity of other immune cells, such as B cells and innate immune cells. T-cell trafficking to sites of infection or inflammation is an important step in T-cell responses. Chemokine and cytokine gradients and increased expression of T-cell chemokine receptors lead to T-cell trafficking. CXC chemokine receptor (CXCR) 3 is the chemokine receptor highly expressed on activated Th1 cells. CXCR3 ligands include CXC chemokine ligand 10 and PF4.102,103 We have found in a mouse model of cerebral malaria that PF4−/− mice have decreased expression of T-cell CXCR3 indicating that PF4 may directly or indirectly mediate CXCR3 expression and T-cell trafficking.2 Other platelet-derived chemokines recruit and activate T cells at sites of vascular inflammation. For example, CC chemokine receptor 5 is a T-cell receptor for both MIP-1α and RANTES (CC chemokine ligand 5), each of which is present at high concentrations in α-granules. T cells may activate platelets through a T-cell CD40L/platelet CD40 interaction leading to platelet RANTES release and further T-cell recruitment.104 Using an acute skin graft model, we have found that platelets increase T-cell graft infiltrates and rejection, in part via platelet-derived thromboxane.41,105 Prostaglandins such as thromboxane also facilitate Th1 differentiation and Th17 expansion contributing to the development of inflammatory diseases,56 but the relevance of platelet-derived prostaglandins to T-cell development and differentiation remains to be determined.
Platelets are the major source of soluble CD40L (soluble CD40L/soluble CD154). Platelet CD40L is stored in α-granules, and with activation CD40L becomes expressed on the platelet surface or released in soluble form into the extracellular environment. In a cardiac allograft model, platelet-derived soluble CD40L was sufficient to initiate rejection independent of other CD40L cell sources.14 Platelet-derived CD40L can also be delivered by MPs increasing the number and distance of interactions between platelets and other cells.106 Platelet CD40L augments T-cell immunity to viral challenge and is necessary for optimal production of immunoglobulin G by inducing DC maturation and B-cell isotype switching.107,108 It has also been suggested that when antigen-specific B and T cells are rare, platelets enhance signals needed for adaptive humoral immunity and germinal center formation.108
We have recently discovered in a mouse cardiac transplant model that platelets have a central role in maintaining CD4+ Th cell homeostasis and regulating Th differentiation into effector Th subtypes.109 Platelet-derived PF4 is needed to limit Th17 differentiation and expansion, both basally and following immune stimulation. PF4 effects on Th differentiation may be mediated in part by PF4 limiting TGF-β signaling. These findings greatly expand our understanding of how platelets shape acquired immune development and indicate that platelets help maintain basal Th cell balance.
Platelets influence adaptive immunity through modulation of DC functions. Platelets may recruit and activate DCs through interactions between DC-derived CD11b/CD18 (Mac-1) and platelet junctional adhesion molecule C, thereby increasing DC activation.110 DC expression of T-cell costimulatory molecules CD80 and CD86 is increased by activated platelets in a contact-independent manner111 leading to a stronger and more rapid T-cell response. Platelets also direct pathogen delivery to DCs. Listeria monocytogenes associates with platelets in the blood in a GPIb- and complement-dependent manner leading to targeting of the platelet-Listeria complexes to splenic CD8α+ DCs.112 The result may be a directing of bacterial clearance away from less immunogenic phagocytes to the more immunologically active CD8α+ DCs.
Although it is clear that platelets affect all phases of immune responses, more work remains to fully appreciate how platelets directly and indirectly affect acquired immune responses. Because T cells, and CD4+ T cells in particular, are master regulators of the immune system, a better understanding of platelet and T-cell interactions is likely to impact a broad range of inflammatory and immune conditions.
Platelets and blood vessel interactions
Because of the great health impact of atherosclerosis, much has been described regarding interactions between platelets and endothelial cells. Atherosclerosis is now clearly defined as an inflammatory disease driven by immune cell interactions with the vessel wall.113 Many reviews have described the activities of platelets at the interface with endothelial cells and atherosclerosis,114 so we will focus on emerging new investigations demonstrating that platelets are mediators of intercellular vascular communication via platelet-derived MPs, and the transfer of platelet-derived microRNA (miRNA) to other vascular cells, because each has been linked to the pathogenesis of vascular inflammation and directly impacts immune cell trafficking.
MPs are released by almost all types of cells. Activated platelets release MPs, and platelets are a major source of circulating MPs.115-117 MPs are commonly defined as lipid membrane vesicles 0.1-1 µM in size. An increased number of circulating platelet MPs (PMPs) correlates with the development of atherosclerosis in patients with diabetes,118 the amount of heart tissue at risk in recent myocardial infarction,119 and the development of acute coronary syndrome and stroke.120-122 PMPs carry adhesion molecules (such as P-selectin) and chemokines (such as RANTES), facilitating monocyte arrest at the site of PMP deposition along the vessel wall, potentially accelerating atherosclerosis.123 Developmental endothelial locus-1 contributes to endothelial uptake of PMPs because Del-1−/− mice had decreased PMP uptake and increased circulating PMPs in an LPS challenge model.124 Statin usage decreases the number of PMPs, potentially providing a potential non-lipid-lowering protective effect of statins.125 The inflammatory effects of PMPs extend to other diseases. PMPs are present at increased numbers in the joint space of individuals with rheumatoid arthritis and are proinflammatory by inducing synovial fibroblast cytokine responses in a PMP IL-1α- and IL-1β-dependent manner.54 PMP IL-1β also contributes to endothelial permeability during dengue virus infection.126,127
MPs carry miRNAs, which are small RNAs ∼20-22 nucleotides in size that regulate gene expression. Each miRNA has the potential to alter the expression of hundreds of genes, increasing the potential impact that platelet miRNA transfer may have on cardiovascular disease. Increased circulating miRNA has been noted in both coronary artery disease and post myocardial infarction,128,129 and recent evidence has suggested that platelet-derived miR-340 and miR-624 are upregulated in patients with premature atherosclerosis.130 Platelets contain >250 different identified and quantified miRNAs.130-133 Platelet RNA and miRNAs can be functionally transferred to other cell types via PMPs. In an in vitro system, RNA from PMPs altered THP-1 (monocyte cell line) gene expression.134 Recent studies have indicated that platelets transfer miRNA in vivo by activated platelet release of PMP-associated miRNA. PMP-derived Ago2 complexes may be functional and taken up by endothelial cells, regulating endogenous endothelial cell gene expression.135 Platelets activated during myocardial infarction release miR-320b that is taken up by endothelial cells and may regulate intercellular adhesion molecule 1 expression, leukocyte adhesion, and trafficking.136 These emerging studies indicate that platelet miRNAs mediate vascular inflammatory processes in a transcellular manner.
Platelets and infectious disease
Infectious disease continues to be a leading cause of death globally. Platelets have been associated with vascular inflammatory and immune complications of malaria, sepsis, HIV, and influenza, each of which causes a large public health burden worldwide. Our knowledge of how platelet-pathogen interactions help dictate infectious disease outcomes has rapidly expanded. Studies have indicated a complicated role for platelets in infectious disease pathogenesis; platelets may help limit the growth of many organisms, but platelet-mediated inflammatory responses may complicate disease progression.
Malaria is caused by the mosquito-transmitted Plasmodium parasite. Despite extensive research over many decades, malaria continues to be a significant cause of death globally and has a major economic impact.137 In uncomplicated malaria, symptoms are typically secondary to red blood cell (RBC) destruction and anemia. Thrombocytopenia is noted very early following infection,138-140 and platelet counts return to normal after parasite clearance.141 The cause of the thrombocytopenia (platelet activation and clearance vs spleen sequestration vs lack of production) remains unclear. We have found evidence for platelet activation within 24 hours of infection in a mouse model.51 Plasmodium-induced immune activation and inflammatory cell–mediated tissue injury can lead to vascular compromise that complicates disease progression. This is commonly referred to as severe malaria. Children are at particular risk for cerebral vascular injury following malaria infection (cerebral malaria). In one study, up to 33% of children admitted to a hospital in Kenya had malaria infection, and of those 47% had evidence of neurologic deficits142 demonstrating the clinical importance of gaining a better understanding cerebral malaria pathogenesis. Cerebral vascular thrombosis and multifocal strokelike lesions are often noted on autopsy specimens of those who die of cerebral malaria.143 Many laboratories have demonstrated a central role for platelets in initiating and accelerating this cerebral inflammatory injury,2,144-146 and there is evidence of Plasmodium invasion/uptake by platelets even though the parasite is unable to progress through its life cycle within platelets.147 Grau and van der Heyde have demonstrated that platelets are necessary for the development of experimental cerebral malaria,148-150 leading to a unifying platelet-centered model for its pathogenesis. Grau has proposed that platelet activation induces the expression of endothelial cell adhesion molecules, leading to more platelet-endothelial interactions, infected RBC and leukocyte cerebral vascular localization, and ultimately cerebral vascular compromise.146 Using the murine model of cerebral malaria, we have implicated PF4 as a platelet-derived chemokine that significantly contributes to leukocyte cerebral vascular trafficking.2,151 This has been further validated in clinical studies demonstrating that plasma PF4 is a predictive biomarker of cerebral malaria in humans.152 Other studies have found platelet-mediated endothelial cell destruction both in vitro and in vivo in malaria models, further highlighting the adverse roles for platelets in cerebral malaria.153
There have also been reports that in uncomplicated malaria (limited inflammation and no cerebral vascular compromise) platelets may have a protective role by directly killing Plasmodium, making the role for platelets in malaria infection confusing and dependent on the inflammatory response to infection.154 Using Plasmodium parasite infection models that do not induce severe malaria or a strong immune response, platelet-deficient mice had increased parasitemia, and platelets were shown to kill intra-RBC parasites in vitro.154 The chemokine we found to be detrimental in experimental cerebral malaria, PF4, was shown to have parasite-killing effects through its antimicrobial domain, independent of its chemokine domain in a nonsevere malaria model.155 This highlights both the complexity of platelets in immune responses, particularly in infectious diseases, and the complicated roles for PF4 in health and disease. Based on these studies, we explored whether platelet-driven outcomes are also inflammation and timing dependent in complicated malaria models. Platelets are activated as early as 24 hours after Plasmodium infection both in murine models and in human disease,51,141 a time when the APR is initiated. The APR response to malaria infection is dependent on platelets, and the platelet-induced APR helps limit the early parasite burden.51 This indicates that platelets may have an early protective role in limiting parasite burden, but with continued platelet activation and the release of platelet-derived immune mediators, platelets accelerate cerebral vascular compromise.
With the development of antiviral therapies and the greatly improved survival of HIV+ individuals, cardiovascular complications of chronic HIV infection and the associated drug regimens have become an important and poorly understood clinical consideration. The risk of thrombosis, including deep vein thrombosis and pulmonary embolism, is increased in HIV+ individuals.156,157 The mechanisms are not clear but may involve increased platelet activation, and with it broad inflammatory consequences. Studies using a primate simian immunodeficiency virus infection model have shown that platelets are activated early in infection, contributing to a drop in platelet count immediately following infection.158,159 Furthermore, platelet-monocyte aggregates are greatly increased in the circulation of both infected primates and humans, and platelets may induce monocyte differentiation into a more inflammatory phenotype.159,160 Thrombocytopenia is associated with increased neurologic complications of HIV infection, and neuro-HIV is hypothesized to be driven by infected macrophage migration into the brain.158,160,161 This has led some to speculate that a platelet-induced macrophage inflammatory phenotype may accelerate neurologic complications of HIV infection, but much remains to definitively demonstrate this. Influenza also exerts a large public health impact every year. In the 2009 H1N1A pandemic, ∼6% of patients experienced a thrombotic event.162 Platelet activation in H1N1A infection exceeded platelet activation noted in bacterial pneumonia.163 Similar to HIV infection, platelet-monocyte aggregates are elevated in influenza infection suggesting that platelets affect viral immune responses.163 Many of these important associations are only beginning to be identified. More basic science study is needed to better define mechanistic roles for platelets in driving influenza-related infection complications.
As noted previously in the discussion of platelet TLRs, platelets interact with and may help clear bacterial infections. As early as 1995, it was recognized that thrombin-stimulated platelets facilitated the clearance of adherent Streptococci in experimental infective endocarditis.164 Specific factors such as β-defensins are released from platelets activated by the Staphylococcus aureus α-toxin, impairing S aureus growth and inducing neutrophil extracellular trap formation.165 Platelets may also “nucleate” or help trap blood pathogens on Kupffer cells, the liver-specific macrophage present in hepatic sinusoids in direct contact with circulating blood.166 Bacteria infection of platelet-depleted and GPIbα−/− mice resulted in increased endothelial damage and vascular leak. In addition, these mice had reduced bacteria trapping in the liver demonstrating a potentially important role for platelets and Kupffer cell interactions in clearing bacteria within liver sinusoids. However, similar to the paradigm discussed with malaria, continued platelet activation in response to a systemic bacterial infection (sepsis) may lead to complications and worsen sepsis outcomes.167
As an awareness of the nonhemostatic functions of platelets grows, studies into the role of platelets in infectious diseases are also increasing and are likely to continue to be better appreciated.
Summary
With the continued discovery of exciting new associations between platelets and inflammatory disease, platelets will continue to become better understood and appreciated as an immune cell. Platelet numbers, the diversity of platelet-derived inflammatory mediators, and the potential for multiple interactions between platelets and other cells, both directly and indirectly, increase the impact of platelets on inflammatory conditions, despite their small anucleate status. As we better understand the immune regulatory functions of platelets, we are also likely to better understand the roles for platelets in multiple inflammatory and infectious diseases.
Acknowledgments
This work was supported by grants from the American Heart Association (13EIA14250023) (C.N.M.) and the National Institutes of Health, National Center for Research Resources (TL1 RR024135-05) (L.M.C.).
Authorship
C.N.M., A.A.A., K.L.M., and L.M.C. wrote and researched parts of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Craig Morrell, Box CVRI, 601 Elmwood Ave, Rochester, NY 14642; e-mail: Craig_Morrell@URMC.Rochester.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal