To the editor:
Vaso-occlusion (VOC) in sickle cell disease (SCD) results from many pathophysiological mechanisms including sickling of red blood cells, hemolysis, inflammation, vascular adhesion, and reduced nitric oxide (NO) bioavailability.1 All these mechanisms interact together to trap blood cells and to accentuate the local blood flow decrease and subsequent local hypoxia.
In healthy and pathological conditions, physical training results in physiological and molecular adaptations including anti-inflammatory effects and endothelial activation limitations.2 In SCD, these adaptations could be beneficial by limiting physiological factors involved in the VOC. In previous studies, we demonstrated that physical training blunted plasma soluble vascular cell adhesion-1 (VCAM-1)3 and improved NO bioavailability in sickle cell trait carriers.4 The present study investigated the effects of chronic physical activity at baseline and in response to a VOC stress in the lungs of sickle SAD mice.
Healthy (C57Bl/6J) and SAD male mice were used in this study. The SAD transgene reproduces the hypoxia-induced vaso-occlusive events of human SCD.5 Control mice (CON) lived in standard mouse cages, whereas physical activity mice (PA) were housed in cages equipped with running wheels. After 8 weeks, mice were euthanized either after normoxic conditions or after exposure to an acute 4 hours of vaso-occlusive hypoxic stress followed by 2 hours of reoxygenation in ambient air (HR).
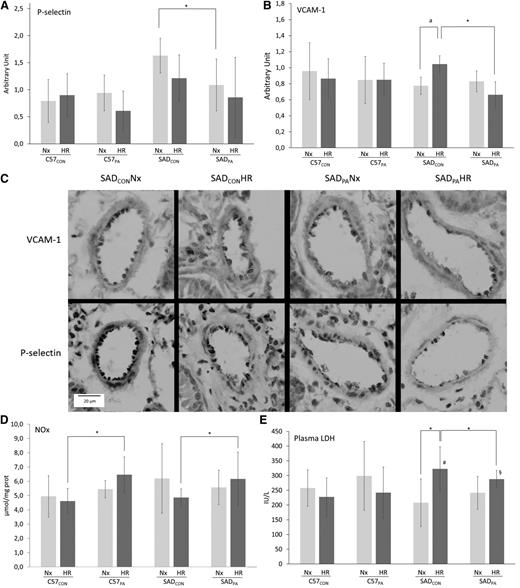
Interestingly, baseline P-selectin expression in the lungs (Figure 1A,C) was more evident in SADCON than in SADPA mice. Because P-selectin is involved in leukocytes and sickle cells trapping initiation, this decreased expression with physical activity may lead to a beneficial reduction in cell aggregate formation and consequently the risk of VOC. Contrary to SADCON mice, the expression of the adhesion molecule VCAM-1 in the PA group was not increased by HR stress (Figure 1B), suggesting that physical activity could limit pulmonary endothelial activation.
Effect of HR stress in PA or CON C57Bl/6 (C57; n = 7 per group) and SAD mice (n = 6 per group). Eight weeks of a voluntary wheel-running protocol were conducted in C57 and SCD SAD mice. Before euthanizing the animals, HR stress (4 hours hypoxic stress at 6.5% oxygen followed by 2 hours of reoxygenation in ambient air) was used to stimulate the pathophysiological parameters known to trigger the VOC in this mouse model. (A) P-selectin and (B) VCAM-1 immunostaining scores in the lung. Immunohistologic slides were blindly semiquantified by 3 experienced anatomists for VCAM-1 and P-selectin with the staining intensity. For each vessel (15 to 20 vessels per mouse), a score from 0 to 3 was attributed (0, no immunostaining; 1, <25% vessel staining; 2, <50% vessel staining; 3, >50% vessel staining). For each mouse, the immunostaining score was the mean of each vessel score from a section. (C) Representative staining for VCAM-1 and P-selectin in the blood vessel of lungs. Magnification ×40. (D) Nitrite/nitrate (NOx) concentrations in the lungs. The Griess method was used: the sum of nitrite and nitrate in the plasma is considered an index of NO production. (E) Plasma concentrations of lactate dehydrogenase (LDH). *P < .05; #, P < .05 vs C57CONHR; §, P < .05 vs C57PAHR; a, P = .07. Values are means ± standard deviation. All variables were tested for normality and variance homogeneity. P-selectin immunostaining and NOx concentrations were tested with nonparametric Kruskall-Wallis test followed by Mann-Whitney U test. The other variables were compared using factorial analysis of variance followed by planned comparisons. Nx, normoxic conditions.
Effect of HR stress in PA or CON C57Bl/6 (C57; n = 7 per group) and SAD mice (n = 6 per group). Eight weeks of a voluntary wheel-running protocol were conducted in C57 and SCD SAD mice. Before euthanizing the animals, HR stress (4 hours hypoxic stress at 6.5% oxygen followed by 2 hours of reoxygenation in ambient air) was used to stimulate the pathophysiological parameters known to trigger the VOC in this mouse model. (A) P-selectin and (B) VCAM-1 immunostaining scores in the lung. Immunohistologic slides were blindly semiquantified by 3 experienced anatomists for VCAM-1 and P-selectin with the staining intensity. For each vessel (15 to 20 vessels per mouse), a score from 0 to 3 was attributed (0, no immunostaining; 1, <25% vessel staining; 2, <50% vessel staining; 3, >50% vessel staining). For each mouse, the immunostaining score was the mean of each vessel score from a section. (C) Representative staining for VCAM-1 and P-selectin in the blood vessel of lungs. Magnification ×40. (D) Nitrite/nitrate (NOx) concentrations in the lungs. The Griess method was used: the sum of nitrite and nitrate in the plasma is considered an index of NO production. (E) Plasma concentrations of lactate dehydrogenase (LDH). *P < .05; #, P < .05 vs C57CONHR; §, P < .05 vs C57PAHR; a, P = .07. Values are means ± standard deviation. All variables were tested for normality and variance homogeneity. P-selectin immunostaining and NOx concentrations were tested with nonparametric Kruskall-Wallis test followed by Mann-Whitney U test. The other variables were compared using factorial analysis of variance followed by planned comparisons. Nx, normoxic conditions.
Endothelial activation has been shown to be notably mediated by decreased NO bioavailability. After HR stress, higher concentrations of NOx were measured in the lungs of SADPA compared with SADCON (+63%, Figure 1D). These results strongly suggest that physical activity could improve pulmonary endothelial function, as already observed in healthy subjects.6 This improvement of NO metabolism may also participate in the decreased VCAM-1 expression observed after HR, via nuclear factor κB inhibition.7 Hemolysis is also known to trigger endothelial activation and vasculopathy.8 After HR, plasma LDH concentrations increased only in SADCON mice, whereas no significant variation was observed in the SADPA group, suggesting that PA could limit HR-induced hemolysis in SAD mice.
In conclusion, this study is the first to demonstrate the beneficial effects of physical activity in response to an HR stress in SCD mice. Similar exercise programs could be a relevant option to prevent pulmonary complications in SCD patients because it has been observed in SCD subjects that moderate acute exercise does not alter their hemorheologic, oxidative stress, or hematologic parameters.9,10 From these results, further studies performed on more severe SCD mice models and in SCD patients will have to confirm the efficiency and/or the benefits of PA in SCD, and to describe the underlying mechanisms of the therapeutic effect of exercise training in SCD.
Authorship
Acknowledgments: The authors thank Pr Y. Beuzard (INSERM U733, Paris, France) for providing initial SAD breeding pairs, which allowed our laboratory to develop a SAD colony; Y. Campion, A. Dorier, and E. Lemarie for technical assistance; and the French Society of Hematology for financial support.
Contribution: E.A., E.C.-S., V.P., and C.M. participated in the design of the study; E.A., A.D., A.R., E.C., G.D.S., A.B., C.F., and V.B. performed the experiments; E.A. and C.M. wrote the manuscript; and E.N.C., E.C.-S., and V.P. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cyril Martin, Center of Research and Innovation on Sports (CRIS EA 647), Université Claude Bernard Lyon 1, Université de Lyon, Campus La Doua, Bat Raphael Dubois, 27-29 bd du 11 novembre 1918, 69622 Villeurbanne Cedex, France; e-mail: cyril.martin@univ-lyon1.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal