In this issue of Blood, Starnes et al describe a novel proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1) mutation that is responsible for pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA) syndrome. This helps us understand how PSTPIP1 regulates podosome and filopodia formation and migration of macrophages.1
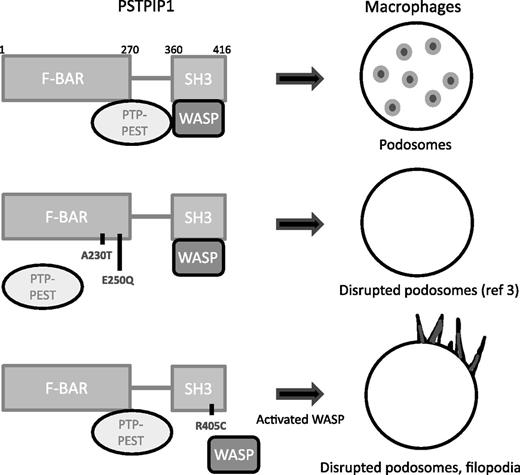
PSTPIP1 interacts with PTP-PEST and WASP. In PAPA patients, two mutations in the F-BAR domain impair the interaction with PTP-PEST. In the article by Starnes et al, a novel mutation was identified in the SH3 domain that affects WASP binding. The consequences of these mutations on macrophage podosomes and filopodia are illustrated.
PSTPIP1 interacts with PTP-PEST and WASP. In PAPA patients, two mutations in the F-BAR domain impair the interaction with PTP-PEST. In the article by Starnes et al, a novel mutation was identified in the SH3 domain that affects WASP binding. The consequences of these mutations on macrophage podosomes and filopodia are illustrated.
PAPA syndrome, first described in 1997, is a rare autosomal dominant disease in which patients show a combination of severe arthritis, skin ulcerations, and acne from an early age. All the lesions are sterile because of excessive autoinflammation involving leukocytes. Arthritis usually begins during early childhood after minor trauma or sporadically. In young adults, joint symptoms tend to decrease, and cutaneous symptoms become more active.2
PAPA syndrome is the result of mutations in the PSTPIP1 gene. PSTPIP1 is an adaptor protein highly expressed in hematopoietic cells that links the plasma membrane to the cytoskeleton. It contains both an F-BAR domain, which has been shown to bind the protein-tyrosine phosphatase (PTP) peptide sequence rich in proline (P), glutamic acid (E), serine (S), and threonine (T) (PTP-PEST), a partner of various cytoskeleton and cell adhesion molecules, and an SRC homology 3 (SH3) domain, which interacts with several proteins, such as the inflammasome regulator pyrin, the tyrosine kinase c-Abl and, critically for the Starnes et al study, with the hematopoietic lineage-restricted Wiskott-Aldrich syndrome protein (WASP) (see figure). WASP is an activator of the actin nucleating complex actin-related protein (Arp)-2 and Arp3, and the scaffold activity of PSTPIP1 makes it possible for PTP-PEST to dephosphorylate WASP.2
The PAPA mutations originally identified in PSTPIP1, A230T and E250Q, were located in the F-BAR domain. They abolish the interaction of PSTPIP1 with PTP-PEST, leading to hyperphosphorylation of PSTPIP1, which was proposed to cause activation of the inflammasome and release of interleukin-1β (IL-1β).2 A previous study from Cortesio et al3 reported that, in patients with those mutations, there was an apparent defect in macrophage migration. This involved defective macrophage chemotaxis to macrophage colony-stimulating factor, an impaired migration in Matrigel invasion chambers, a decreased ability to degrade the extracellular matrix (ECM) protein gelatin, and a significant defect in podosome formation.3
Podosomes are F-actin–rich cone structures surrounded by a ring of adhesion proteins and proteins linking integrins to the cytoskeleton4 that degrade the ECM. They form at the ventral membrane of only a few cell types (macrophages, immature dendritic cells, and osteoclasts). Altered formation of podosomes has been shown to translate into impaired cell adhesion and ECM degradation, defective chemotaxis, and reduced migration in three-dimensional (3D) environments in vitro and in vivo.4,5
In this issue of Blood, a novel mutation in the PSTPIP1 gene is described in a patient with PAPA syndrome.1 The R405C mutation is the first mutation described in the SH3 domain of PSTPIP1. It is distant from the binding domain of PTP-PEST, which normally interacts with PSTPIP1. The patient’s macrophages were found to have very few F-actin dots that did not colocalize with vinculin, a hallmark of podosomes. Instead, they showed prominent filopodia and a highly active matrix-degrading activity, and they were more chemotactic and invasive compared with control cells.
The functional consequences of that mutation were studied in PSTPIP1-depleted THP-1 macrophages expressing the mutant protein. Like the patient’s cells, THP-1 cells expressing PSTPIP1-R405C also exhibited disrupted podosomes and prominent formation of filopodia. Interestingly, WASP activity was enhanced in these cells, which correlated with an impaired interaction of WASP with PSTPIP1-R405C. Thus PSTPIP1-R405C causes WASP relocation away from PTP-PEST. However, WASP hyperphosphorylation could not be detected, suggesting additional regulation mechanisms.
WASP-deficient macrophages do not form podosomes, but constitutively active WASP leads to podosome disassembly.4 The phosphomimetic active form of WASP has varying degrees of impact on podosome formation, depending on the cell type studied.6,7 Introduction of constitutively active CDC42, an activator of WASP, triggers podosome disruption and filopodia formation.4 Taken together, those studies4,6,7 suggest that WASP activity has to be finely tuned to maintain normal podosome assembly and dynamics. This equilibrium is clearly disturbed in macrophages from the R405C patient. It will be interesting to find out how the A230T or E250Q mutations alter WASP activity. In addition to WASP binding, it will be interesting to explore the binding of the other proteins that interact with the SH3 domain of PSTPIP1 to create a broader picture of the general consequences of the R405C mutation.
Furthermore, the study by Starnes et al provides new insight into the role of PSTPIP1 as a regulator of the transition between podosomes and filopodia on 2D substrates. While there are examples of podosome alterations that correlate with inhibited ECM degradation,5,8 here, in the absence of podosomes, the delivery of proteases appeared to be transferred to filopodia with an even more efficient matrix-degrading activity in PSTPIP1-R405C cells than in control macrophages. This suggests that filopodia can substitute for podosomes to degrade ECM proteins. Migration in 3D dense matrices such as Matrigel requires ECM degradation for macrophages to create paths.9 In these environments, macrophages form cell protrusions called 3D podosomes at the tip of which podosome proteins accumulate along with F-actin and matrix proteolytic activity.9,10 The study by Starnes et al provides a very important observation because it shows that the ability of this patient’s cells to migrate in dense matrices is highly efficient despite podosome disruption. It will be interesting to characterize whether filopodia-bearing cells in 2D do form 3D podosome structures in dense matrices.
Mutations in PSTPIP1 that disrupt the interaction with PTP-PEST result in impaired chemotaxis and 3D migration of macrophages, whereas the R405C mutation triggers an opposite phenotype, which would be expected to lead to different clinical outcomes. Indeed, the patient with the R405C mutation showed aggressive pyoderma gangrenosum but no arthritis, whereas arthritis is usually very prominent in patients carrying A230T or E250Q mutations. Those observations suggest that different mechanisms are involved in the two aspects of this autoinflammatory syndrome. Thus, extension of our knowledge about PAPA syndrome mutations may eventually help to adapt therapies. In line with this, therapies targeting IL-1β lack efficacy in some patients2 and the study by Starnes et al may help us understand why.
Conflict-of-interest disclosure: The author declares no competing financial interests.